Preparation method of rare-earth-based fluorite type high-entropy oxide powder material
A technology of oxide powder and fluorite type, applied in the direction of rare earth metal oxides/hydroxides, rare earth metal compounds, chemical instruments and methods, etc., can solve the problem of high reaction temperature, high pressure, complex reaction equipment and preparation process, etc. problem, to achieve the effect of low production cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
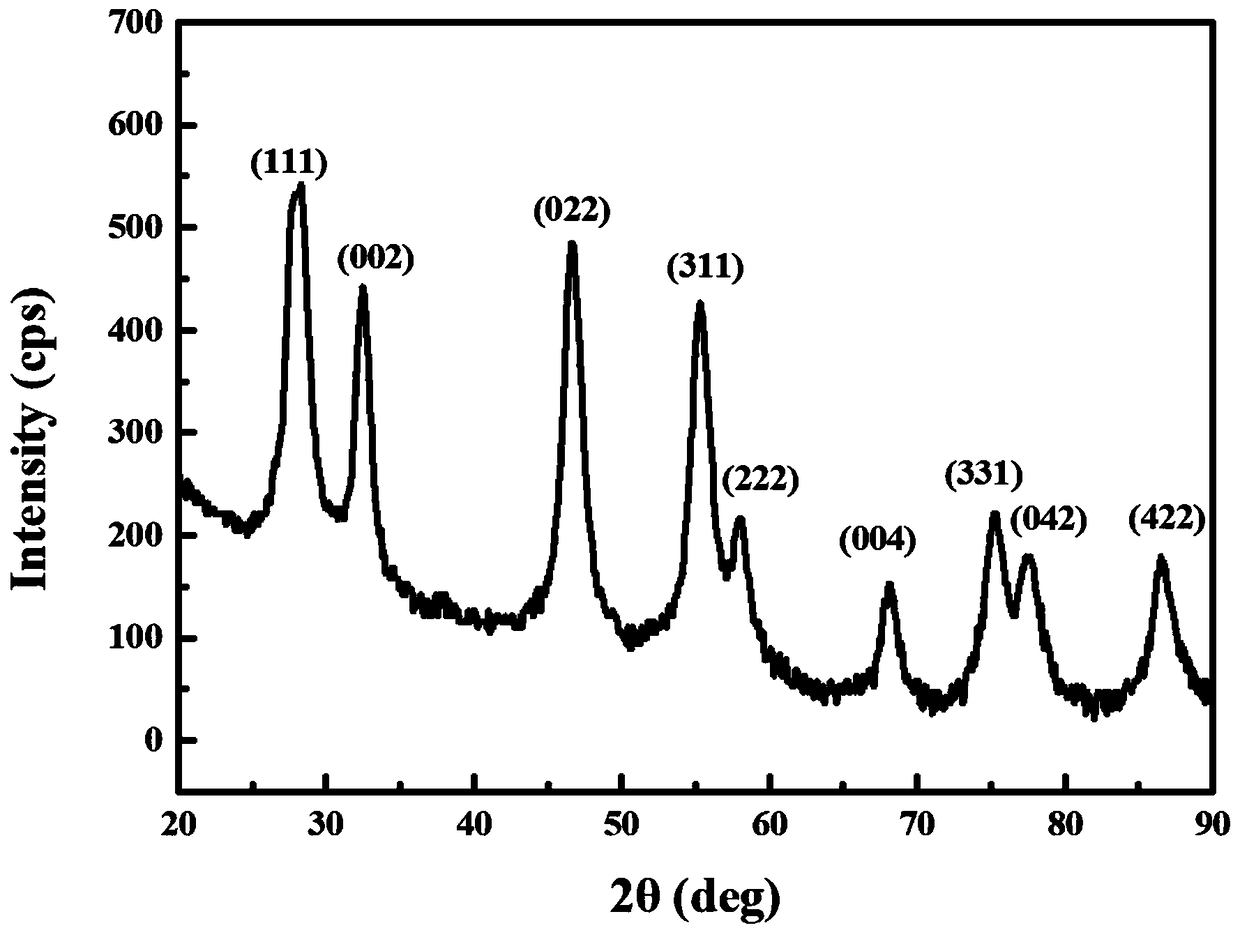
[0026] Weigh 43.42g of Ce(NO 3 ) 3 .6H 2 O, 43.30g of La(NO 3 ) 3 .6H 2 O, 43.50g of Pr(NO 3 ) 3 .6H 2 O, 33.64g of Sm(NO 3 ) 3 .6H 2 O and 38.30g of Y (NO 3 ) 3 .6H 2 O was dissolved in 50mL of distilled water, stirred evenly to obtain a mixed solution containing rare earth nitrate; then 90.09g urea was weighed and added to the above mixed solution, after stirring evenly, the pH of the mixed solution was adjusted to be 7 with ammonia water to obtain a transparent sol; The above-mentioned transparent sol was heated in an oil bath at 130°C to remove the solvent water, and a loose, foamy gel was obtained; finally, the above-mentioned gel was placed in a muffle furnace at 350°C for 30 minutes to obtain a crystal structure of fluorite type ( Such as figure 1 shown), the fluorite-type (CeLaPrSmY)O high-entropy oxide powder material with an average particle size of 35nm (such as figure 2 shown).
Embodiment 2
[0028] Weigh 65.13g of Ce(NO 3 ) 3 .6H 2 O, 67.70g of Gd(NO 3 ) 3 .6H 2 O, 64.95g of La(NO 3 ) 3 .6H 2 O, 62.25g of Pr(NO 3 ) 3 .6H 2 O, 50.46g of Sm(NO 3 ) 3 .6H 2 O and 57.45g of Y (NO 3 ) 3 .6H 2 O was dissolved in 100mL of distilled water, and stirred evenly to obtain a mixed solution containing rare earth nitrates; then 180.15g of acetic acid and 54.02g of oxalic acid were weighed and added to the above mixed solution, and after stirring evenly, the pH of the mixed solution was adjusted to 6 with ammonia water to obtain transparent sol; then heat the above transparent sol in an oil bath at 180°C to remove the solvent water to obtain a loose, foamy gel; finally place the above gel in a muffle furnace at 500°C for 15 minutes to obtain an average Fluorite-type (CeGaLaPrSmY)O high-entropy oxide powder material with a particle size of 80nm (such as image 3 shown).
Embodiment 3
[0030] Weigh 43.42g of Ce(NO 3 ) 3 .6H 2 O, 45.14g of Gd(NO 3 ) 3 .6H 2 O, 43.30g of La(NO 3 ) 3 .6H 2 O, 43.84g of Nd(NO 3 ) 3 .6H 2 O, 43.50g of Pr(NO 3 ) 3 .6H 2 O, 33.64g of Sm(NO 3 ) 3 .6H 2 O and 38.30g of Y (NO 3 ) 3 .6H 2 O was dissolved in 100mL of distilled water, and stirred evenly to obtain a mixed solution containing rare earth nitrate; then 42.04g of urea and 42.00g of acetic acid were weighed and added to the above mixed solution, and after stirring evenly, the pH of the mixed solution was adjusted to 8 with ammonia water. Obtain a transparent sol; then heat the above transparent sol in an oil bath at 130°C to remove the solvent water to obtain a loose, foamy gel; finally place the above gel in a microwave oven with a microwave power of 600W for 5 minutes to obtain Fluorite-type (CeGaLaNdPrSmY)O high-entropy oxide powder materials with an average particle size of 120nm (such as Figure 4 shown).
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com