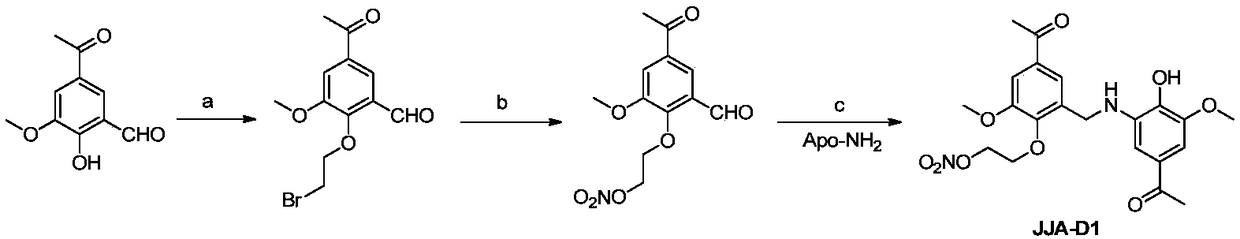
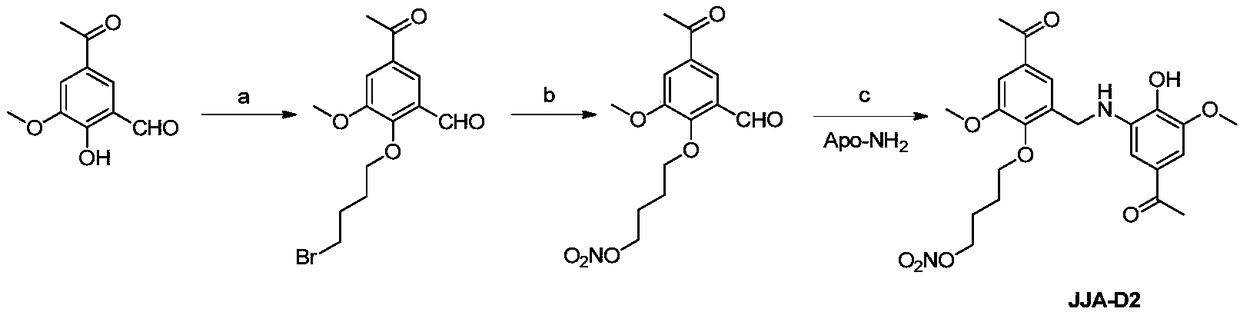
Derivative of Kutkin dimer analog JJA-D0 or its pharmaceutically acceptable salt, preparation method and use thereof
A technology of JJA-D0 and berberine, applied in the field of medicine, can solve the problem of single site that can be modified by the parent compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
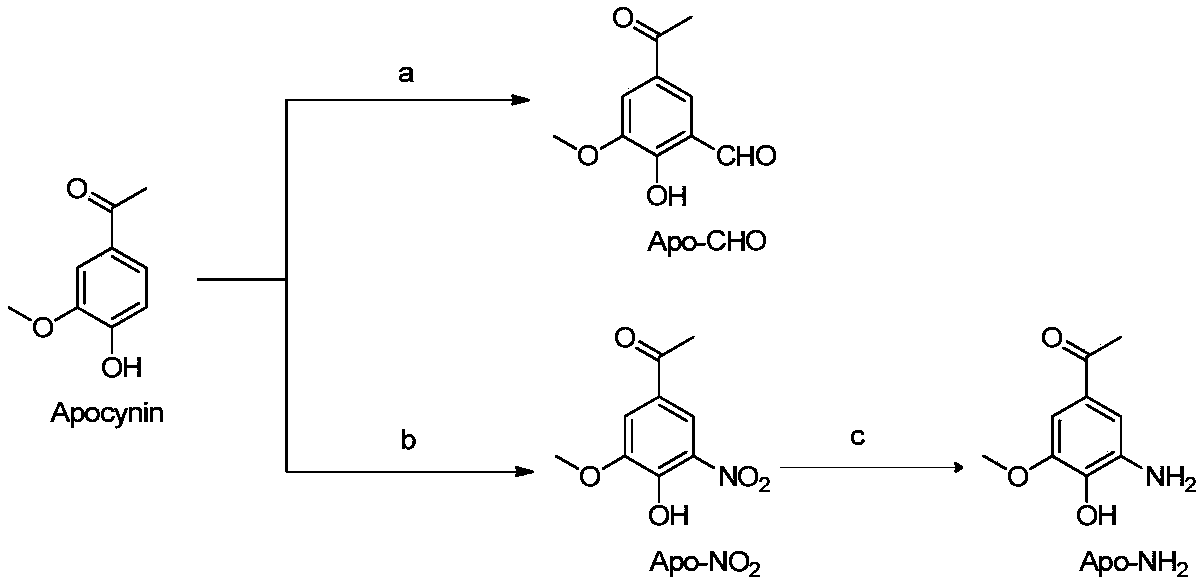
[0085] Embodiment 1: Compound Apo-NO 2 Synthesis
[0086] Take berberine (1.66g, 10mmol), add 10ml of glacial acetic acid to dissolve, slowly add 1.5ml of 67wt% concentrated nitric acid under ice bath stirring, react for 30min, remove ice water, react at room temperature for 3h, add to the system after the reaction Ice-water, suction-filtered to obtain a yellow solid, washed with ice-water, and recrystallized in 95% (v / v) ethanol to obtain yellow needle-like crystals (1.65 g, yield 78%). This compound is a known compound, which is consistent with the spectra recorded in the literature through mass spectrometry and nuclear magnetic spectrum.
Embodiment 2
[0087] Embodiment 2: Compound Apo-NH 2 Synthesis
[0088] Apo-NO 2 (2.11g, 10mmol) was dissolved in 30ml ethanol, and the mass ratio was added to be 10% Pd / C (950mg), and the reaction mixture was passed into H 2 Reacted overnight, washed with water after the reaction, took the organic layer and concentrated it under reduced pressure, and separated it on a silica gel column (ethyl acetate:petroleum ether=1:2) to obtain a light white solid Apo-NH 2 (1.32 g, 73% yield). This compound is a known compound, which is consistent with the spectra recorded in the literature through mass spectrometry and nuclear magnetic spectrum.
Embodiment 3
[0089] Embodiment 3: the synthesis of compound Apo-CHO
[0090] Put berberine (1.66g, 10mmol) in a flask, add a mixed solution of 3ml of triethylamine and 20ml of absolute ethanol, then drop 30ml of 50wt% sodium hydroxide aqueous solution into the system, stir and mix. Chloroform (5.95g, 50mmol) was added dropwise to the reaction solution, and reacted at 70-80°C for 2h. The reaction solution was cooled to room temperature and acidified to pH=1 with hydrochloric acid. The organic layer was collected, the aqueous layer was extracted with dichloromethane, the organic layers were combined and washed with water, dried over anhydrous sodium sulfate, concentrated, and separated on a silica gel column (ethyl acetate:petroleum ether=2:3) to obtain a light yellow powder Apo-CHO (0.69 g, 36% yield). This compound is a known compound, which is consistent with the spectra recorded in the literature through mass spectrometry and nuclear magnetic spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com