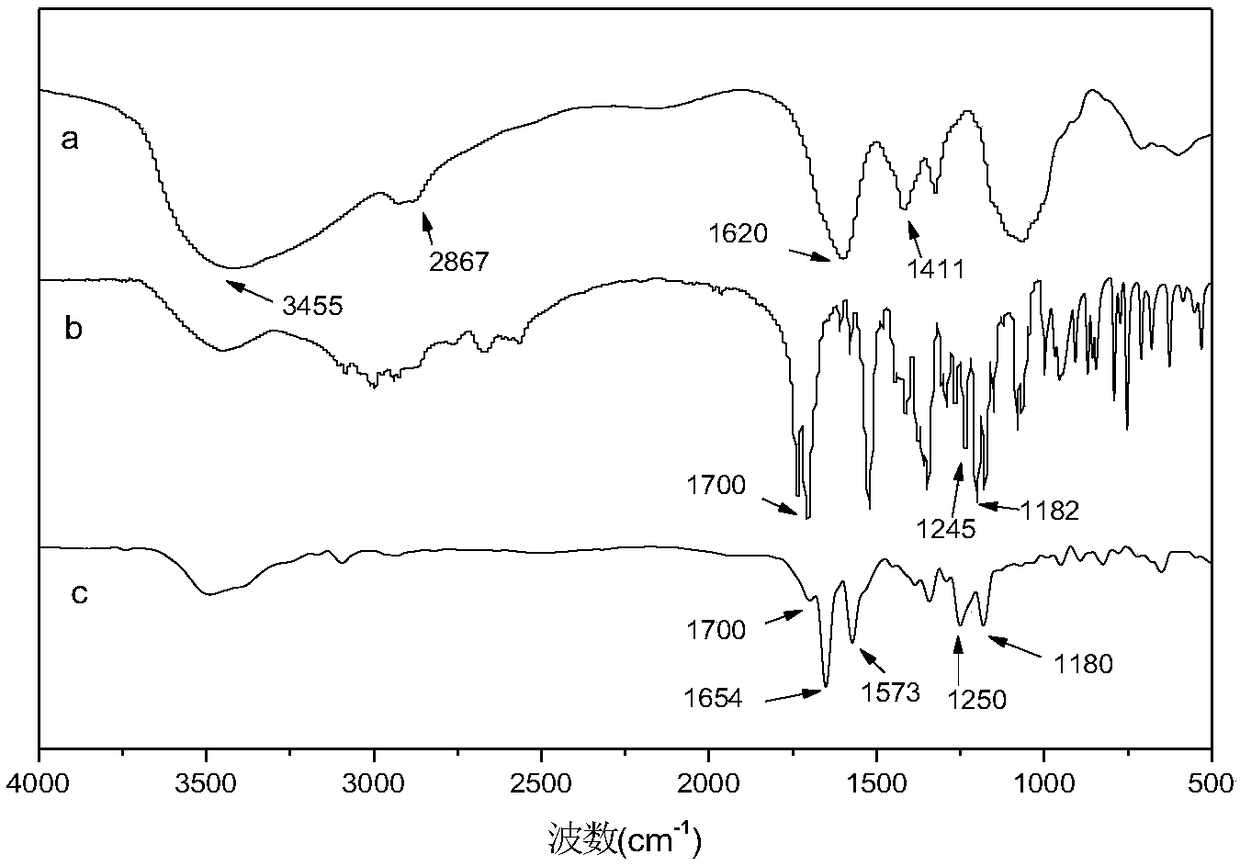
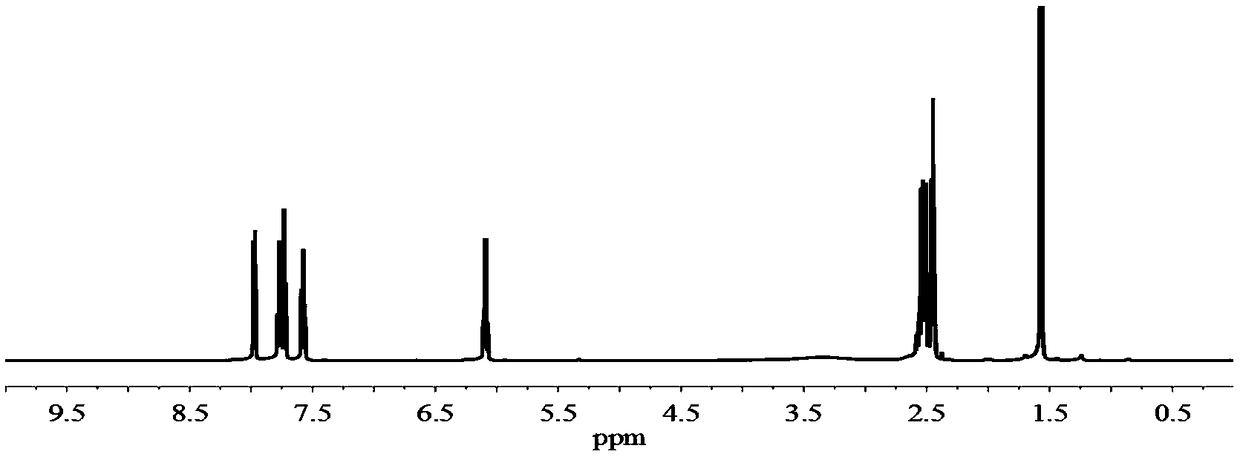
Near-infrared light response photodynamic drug controlled release system and preparation method thereof
A near-infrared light and drug controlled release technology, applied in the field of medicine, can solve the problems of large toxic and side effects and low practical value, and achieve the effects of improving effective concentration, enhancing response ability and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of carboxymethyl chitosan (CMCS):
[0041] Weighing 10g of chitosan with a degree of deacetylation of 90% is added to a 250mL there-necked flask, added 100mL of pure isopropanol, stirred at room temperature for 6h and filtered, took out the filter residue and introduced it into a beaker, then added 60g of NaOH solution (50wt %), fully stirred evenly, refrigerated overnight at -4°C, took it out the next day and waited for natural melting, then transferred to a 500mL three-necked bottle, added 100mL pure isopropanol, and stirred evenly. Get 30g of isopropanol solution (50wt%) of chloroacetic acid and add it dropwise in the three-necked flask, and the dripping is completed in about 2 hours. At this time, the three-necked bottle is in a white emulsified state, and the stirring reaction is continued for 20 hours. The reaction is carried out in an ice bath, and the reaction temperature is controlled. Below 10°C, filter, dissolve the filter cake in 250mL deionized ...
Embodiment 2
[0049] (1) Preparation of carboxymethyl chitosan:
[0050] The preparation method is the same as Example 1, the deacetylation degree of the chitosan used as raw material is 95%; the carboxymethyl substitution degree of the obtained carboxy chitosan is 0.85.
[0051] (2) Preparation of phototrigger compound succinic acid monoester:
[0052] The trigger molecules o-nitrobenzyl alcohol (25.0 mmol), succinic anhydride (5.03 g) and DMAP (1.53 g) were dissolved in pure chloroform (90 mL). The reaction mixture was refluxed for 24h under nitrogen protection. Chloroform was then distilled off under reduced pressure. The mixture was washed three times with 10% hydrochloric acid by volume to remove DMAP, and then washed with saturated NaHCO 3 Solution extraction. The aqueous phase was washed with diethyl ether and adjusted to pH 5.0 with 10% hydrochloric acid by volume. The white precipitate was collected and dried under vacuum at 40°C to obtain o-nitrobenzyl succinic acid monoester...
Embodiment 3
[0058] (1) Preparation of carboxymethyl chitosan:
[0059] The preparation method is the same as Example 1, the deacetylation degree of the chitosan used as raw material is 100%; the carboxymethyl substitution degree of the obtained carboxy chitosan is 1.0.
[0060] (2) Preparation of phototrigger compound succinic acid monoester:
[0061] The trigger molecule 1-pyrenemethanol (25.0mmol), succinic anhydride (5.03g) and DMAP (1.53g) were dissolved in pure chloroform (90mL). The reaction mixture was refluxed for 24h under nitrogen protection. Chloroform was then distilled off under reduced pressure. The mixture was washed three times with 10% hydrochloric acid by volume to remove DMAP, and then washed with saturated NaHCO 3 Solution extraction. The aqueous phase was washed with ether and adjusted to pH 5.0 with 10% hydrochloric acid by volume. The white precipitate was collected and dried under vacuum at 40°C to produce 1-pyrenemethylsuccinate (PMS). All the above reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com