A kind of lithium ion battery cathode material additive and its cathode material and lithium ion secondary battery
A technology for lithium ion batteries and positive electrode materials, applied in the field of electrochemistry, can solve the problems of difficult removal of impurity ions, unsuitability for mass preparation, and impact on battery performance, and achieves good market prospects, good rate performance, cycle stability, and high capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
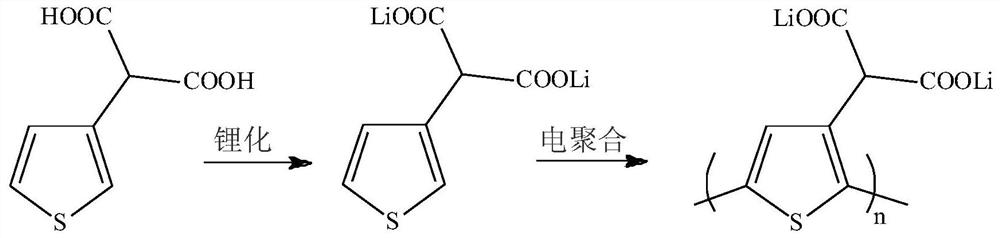
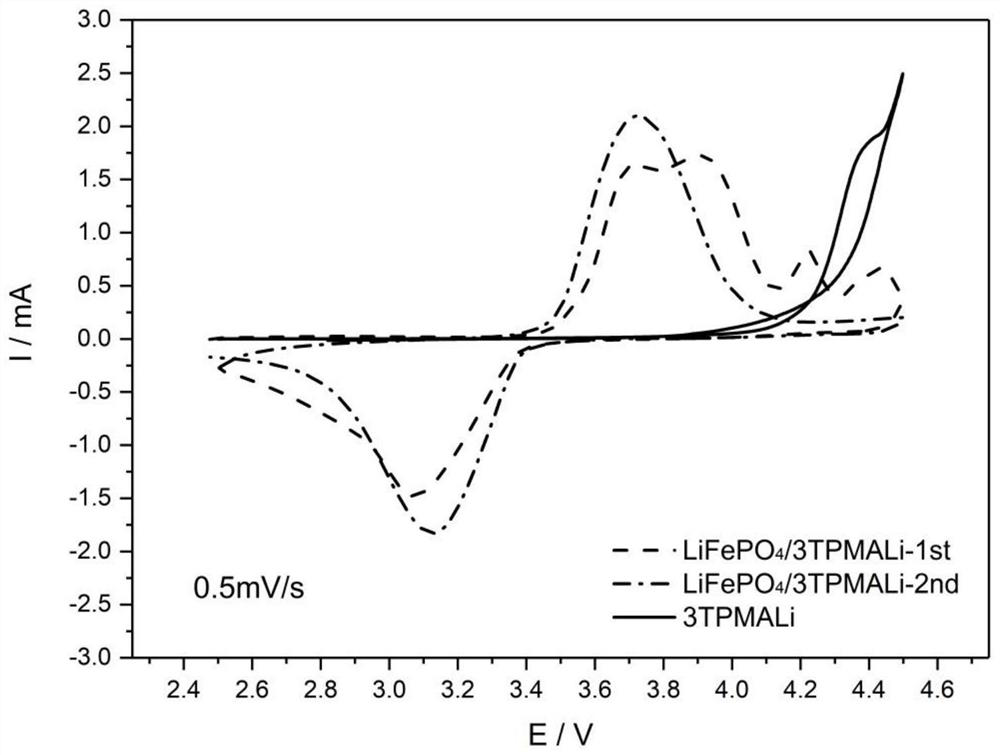
[0038] This embodiment is a lithium ion battery positive electrode material additive and its positive electrode and lithium secondary battery. The additive is a conductive polymer monomer unit containing a high concentration of carboxyl groups. This example selects 3-thiophene malonic acid (3TPA) for use, and reaction course is as follows figure 1 shown.
[0039] The lithiation process of the above-mentioned 3-thiophene malonate is specifically as follows:
[0040] Lithiation of 3-thiophene malonate: 0.8618g (0.01mol) 3-thiophene malonate and 0.4800g (0.02mol) lithium hydroxide (LiOH) were lithiated in solvent water, and the lithiation time was 3 hours. After the lithiation reaction was completed, the solvent was evaporated to dryness to obtain 3-thiophene malonate lithium (3TPMALi).
[0041] The preparation steps of the lithium-ion battery cathode material of the present embodiment are as follows:
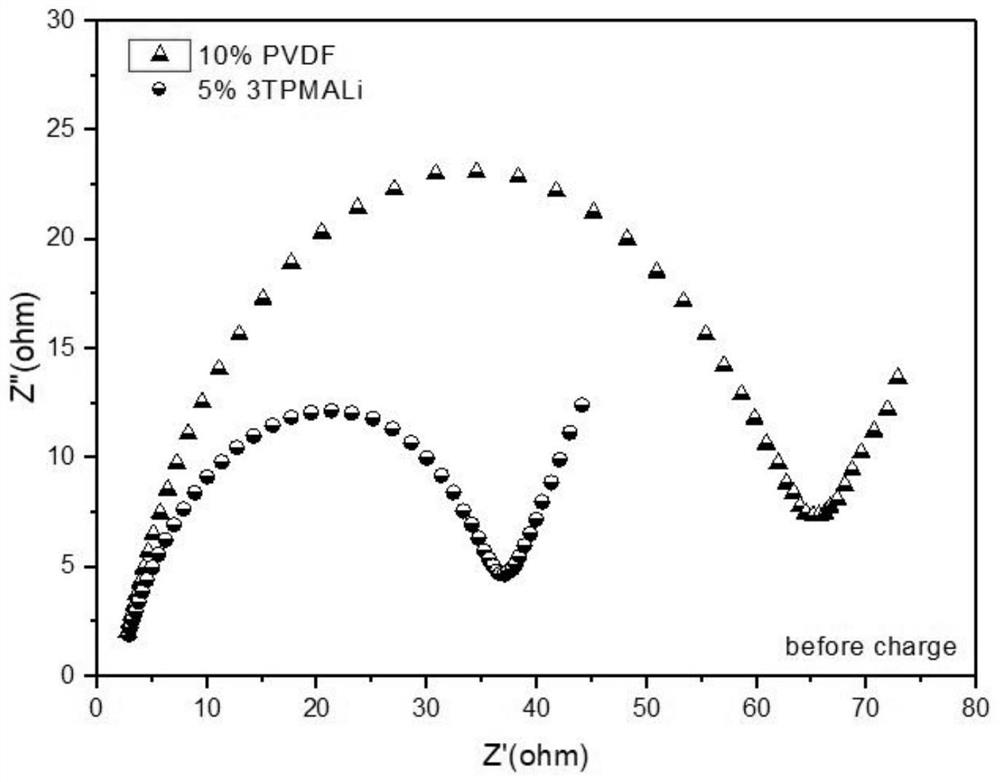
[0042] 0.1402g lithium iron phosphate (LiFePO 4 ), 0.0401g acetylene black,...
Embodiment 2
[0063] This embodiment is a lithium ion battery positive electrode material additive and its positive electrode and lithium secondary battery. The additive is a conductive polymer monomer unit containing a high concentration of carboxyl groups, specifically after lithiation of 3-thiopheneacetic acid monomer Lithium 3-thiophene acetate is formed, which is then prepared by electrochemical in situ polymerization. The structural formula of the 3-thiopheneacetic acid monomer is shown in formula B in the summary of the invention.
[0064] The lithiation process of the above-mentioned 3-thiopheneacetic acid monomer is specifically as follows:
[0065] Lithiation of 3-thiopheneacetic acid monomer: 1.4205g (0.01mol) 3-thiopheneacetic acid monomer and 0.2403g (0.01mol) lithium hydroxide (LiOH) were dissolved in water for lithiation, and the lithiation time was 3 hours. After the lithiation reaction is completed, the solvent is evaporated to dryness to obtain 3-thiopheneacetic acid mono...
Embodiment 3
[0071] This embodiment is a lithium ion battery positive electrode material additive and its positive electrode and lithium secondary battery. The additive is a conductive polymer monomer unit containing a carboxyl group, specifically formed by lithiation of N-pyrrole propionic acid monomer Lithium N-pyrrole propionate is prepared by electrochemical in situ polymerization. The structural formula of the N-pyrrole propionic acid monomer is shown in formula C.
[0072] The lithiation process of the above-mentioned N-pyrrole propionic acid is specifically as follows:
[0073] Lithiation of N-pyrrolepropionic acid: Dissolve 1.3903g (0.01mol) N-pyrrolepropionic acid and 0.2401g (0.01mol) hydroxide in water for lithiation. The lithiation time is 3 hours. After the lithiation reaction is completed, Evaporate the solvent to obtain lithium N-pyrrole propionate.
[0074] The preparation steps of the lithium ion battery positive electrode material of the present embodiment are as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com