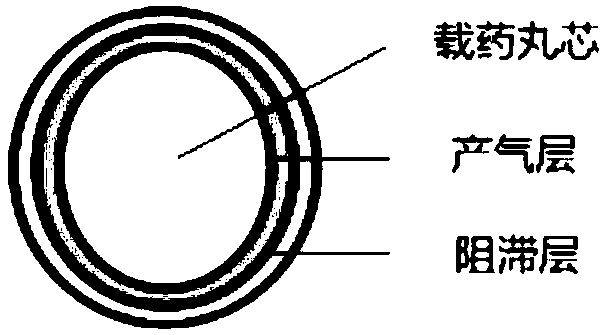
Preparation method of mosapride citrate stomach buoyant sustained-release pellet
A technology of mosapride citrate and sustained-release pellets is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc. It is not good, and the local drug concentration is high, so as to achieve the optimization of gas producing layer and retardation layer, good slow release characteristics, and the effect of expanding production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A method for preparing mosapride citrate gastric floating sustained-release pellets, characterized in that it comprises the steps of:
[0064] 1) Preparation of soft materials
[0065] 1.1) Place stearyl alcohol in ethanol solution, and obtain mixture A after completely dissolving;
[0066] The concentration of the ethanol solution is 95%;
[0067] Get described stearyl alcohol 13g, dissolve completely with above-mentioned ethanol solution 220g;
[0068] 1.2) Mix mosapride citrate, microcrystalline cellulose, sodium bicarbonate and the mixture A obtained in step 1.1), and sieve to obtain mixture B;
[0069] Described mosapride citrate, microcrystalline cellulose, sodium bicarbonate weight are respectively 1g, 25g, 3.4g;
[0070] The sieve used in the sieving process is 80 mesh;
[0071] 1.3) Shake the mixture B until it is evenly mixed, then add an aqueous solution of hydroxypropyl methylcellulose to the mixture B to prepare a soft material;
[0072] The mass fract...
experiment example 1
[0103] Randomly take 100 pellets and place them in 900ml of hydrochloric acid solution with a concentration of 0.1mol / L, at 37±0.5°C, and the rotation speed of the stirring paddle is 75rpm;
[0104] The time required for observing the floating rate to be higher than 90% (floating time) was less than 1min, and the floating rate of the pellets was 84.32% in 8h; the time to record the floating rate of the pellets higher than 80% was more than 10h.
experiment example 2
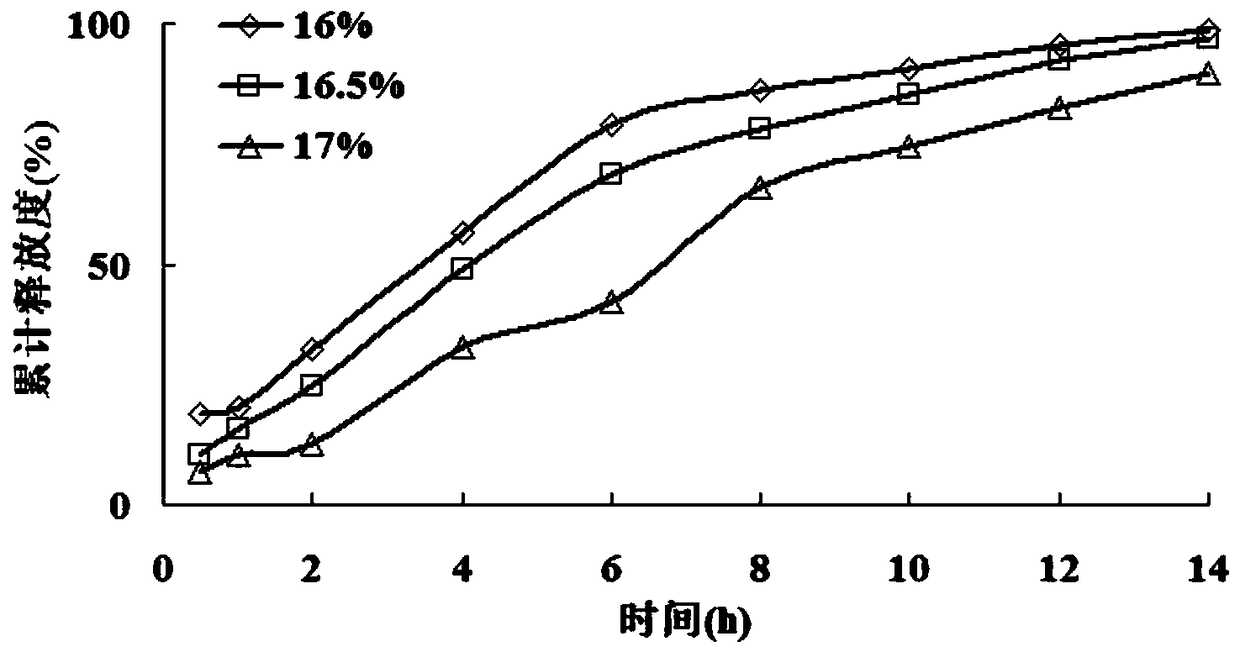
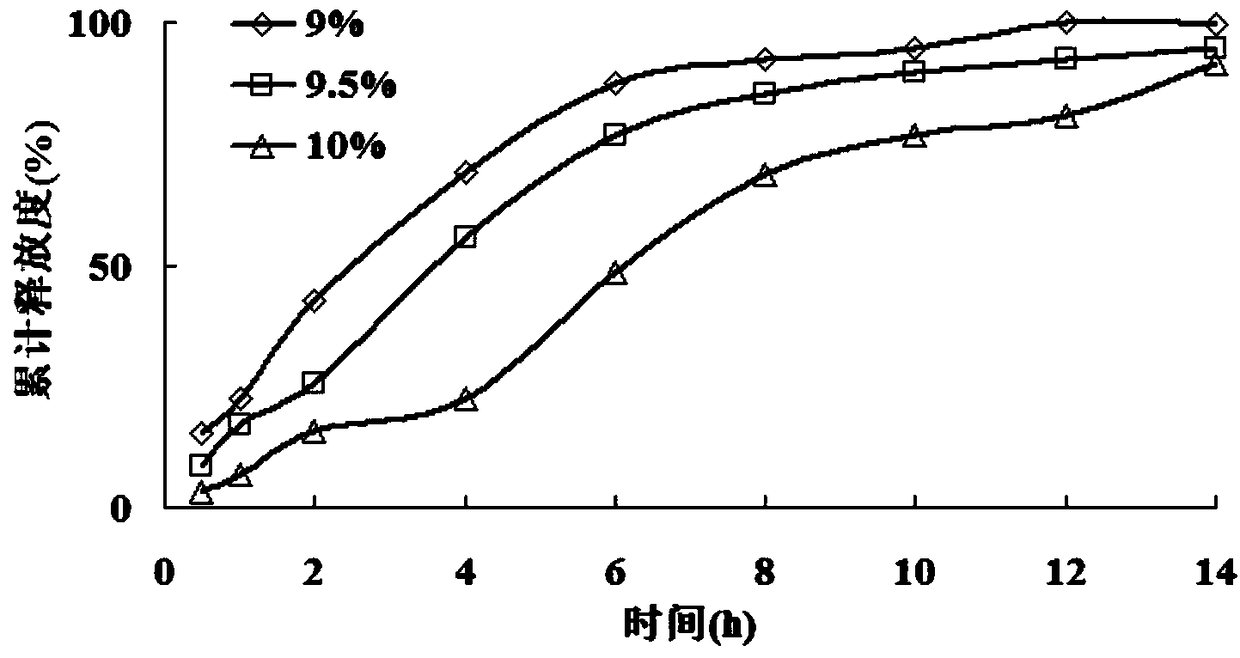
[0106] According to the scheme in Example 1, 3 batches of mosapride citrate gastric floating sustained-release pellets were prepared, and the release rate was measured, specifically: a method for determining the content of mosapride citrate was established by using high performance liquid chromatography Determination of the release rate of the coated sustained-release pellets.
[0107] The chromatographic column is WelchromTMC18 (5μm, 4.6×250mm), the mobile phase is acetonitrile-0.2% phosphoric acid solution (30︰70, v / v), the detection wavelength is 274nm, the column temperature is 30°C, and the flow rate is 1ml / min. Volume: 30 μl.
[0108] The peak eluting time of mosapride citrate was 8.6min, and other excipients did not affect the determination of mosapride citrate.
[0109] After the methodological verification of linearity, precision and recovery, the standard curve equation was established, and the in vitro release of the coated pellets was determined by the rotating ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com