Flue gas desulfurization and denitrification method based on coupling conversion of sodium nitrite method and circulating sodium alkali method
A desulfurization, denitrification, and sodium-alkali technology, which is applied in the field of flue gas desulfurization and denitrification, and can solve problems such as affecting the quality of desulfurization by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
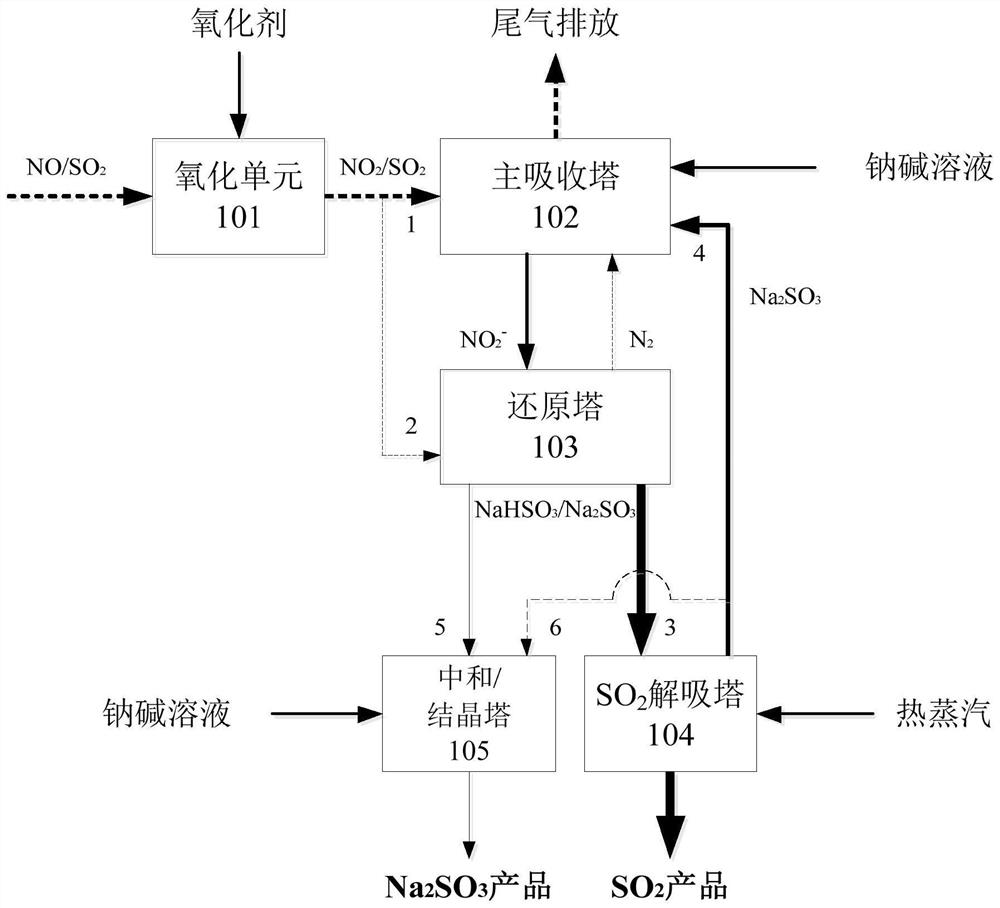
[0038] Such as figure 1 Said, the flue gas desulfurization and denitrification method based on the coupling of the sodium nitrate method and the circulating sodium alkali method comprises the following steps:
[0039] The first step, to the flue gas (the main components are NO and SO 2 ) into the oxidant (choice of ozone or sodium chlorite), and NO is oxidized to NO in the oxidation unit 101 2 ;
[0040] Second step, utilize sodium alkali solution (select mass concentration to be the sodium hydroxide of 5%-30%) in the main absorption tower 102 to the NO in flue gas 1 2 and SO 2 Carry out simultaneous absorption and use higher concentration of sulfite to control the absorbed nitrogen oxides in the state of nitrite;
[0041] In the third step, sodium sulfite and nitrite in the main absorption tower 102 are accumulated until the concentration of nitrite is greater than 0.5% or the pH value of the solution is less than 7 (with the first index as the control standard), and part...
Embodiment 2
[0048] Transfer 100mL of the mixed solution of sodium sulfite and sodium nitrite obtained after absorbing for a certain period of time in the example to a new absorption experimental device, and feed 3000ppmSO into the mixed solution absorption bottle at a flow rate of 500mL / min at 30°C. 2 , the results show that sodium sulfite solution can still further absorb SO in simulated flue gas 2 , and SO 2 The removal efficiency was 95%. After absorbing for a period of time, the absorption liquid was analyzed by ion chromatography, and the result showed that the concentration of nitrite in the absorption liquid decreased significantly. Since NO and NO were not detected in the absorption tail gas 2 , which shows that the nitrite in the mixed absorption solution is not converted into NO and NO 2 The form is released again, but is released from the mixed absorption solution after being reduced to nitrogen.
Embodiment 3
[0050] Prepare 100mL of 10% sodium sulfite solution and feed 3000ppm SO at a flow rate of 500mL / min at 30°C 2 Until breakthrough, the ion analysis product is mainly sodium bisulfite. Heat the saturated solution to about 150°C under the condition of 0.5MPa atmospheric pressure, and blow it off with nitrogen for 60 minutes, and the tail gas is monitored as a high concentration of SO 2 Then continue to carry out ion chromatographic analysis to the absorption liquid, the result shows that part of the sodium bisulfite in the absorption liquid has been converted into sodium sulfite, and its concentration is about 50% of sodium bisulfite in the saturated absorption liquid. This shows that part of the sodium bisulfite in the saturated absorption liquid has been regenerated into sodium sulfite. The regenerated absorption solution continues to be effective against SO with a concentration of 3000ppm 2 The gas is absorbed, and its desulfurization efficiency is still above 99%. This sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com