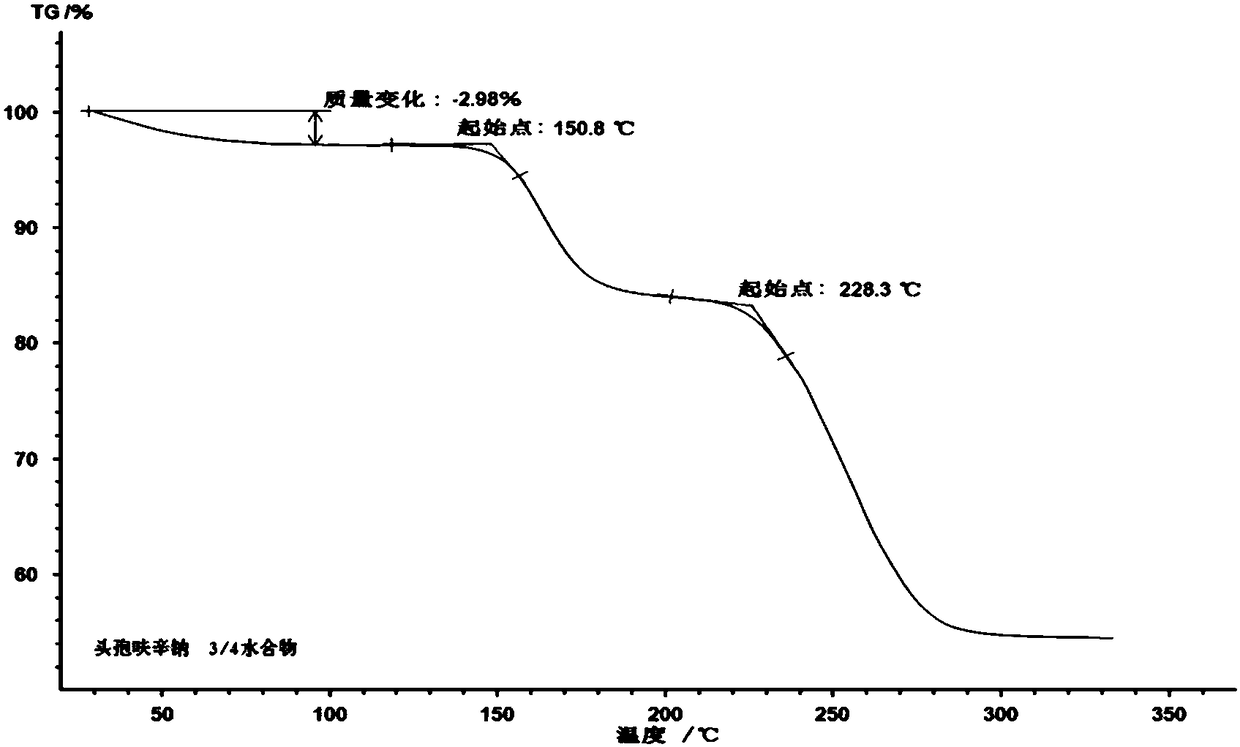
Cefuroxime sodium 3/4-hydrate compound
A technology of cefuroxime sodium and furoxin sodium, which is applied in the direction of organic chemistry, organic chemical methods, organic active ingredients, etc., can solve the problems of low thermal decomposition temperature, poor fluidity, and unclearness, and achieve wide application prospects and thermal stability Good performance and fast drying effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]The preparation of embodiment 1 3 / 4 water cefuroxime sodium compound
[0032] (1) At room temperature, add 852g of cefuroxime acid into 4L of methanol, stir, and slowly add sodium lactate (302g) methylene chloride (604ml) solution to react until the pH value is 6.2;
[0033] (2) Add 4L of ethanol for elution and crystallization, filter, wash with ethanol, and vacuum-dry at 45° C. for 40 minutes to obtain 833 g of cefuroxime sodium 3 / 4 water compound.
[0034] Test results:
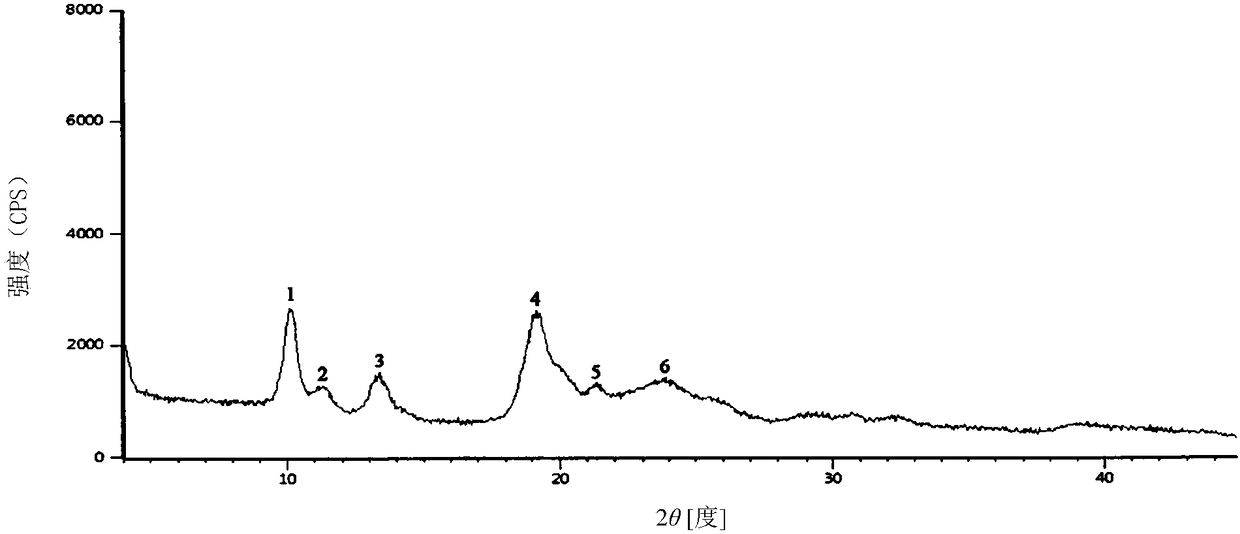
[0035] The X-ray diffraction pattern has characteristic diffraction peaks at the diffraction angles 2θ of 10.18°, 11.38°, 13.34°, 19.06°, and 19.25°, and the relative diffraction intensities of the diffraction angles are 100, 20.51, 38.46, 95.47, 93.47, and 10.48;
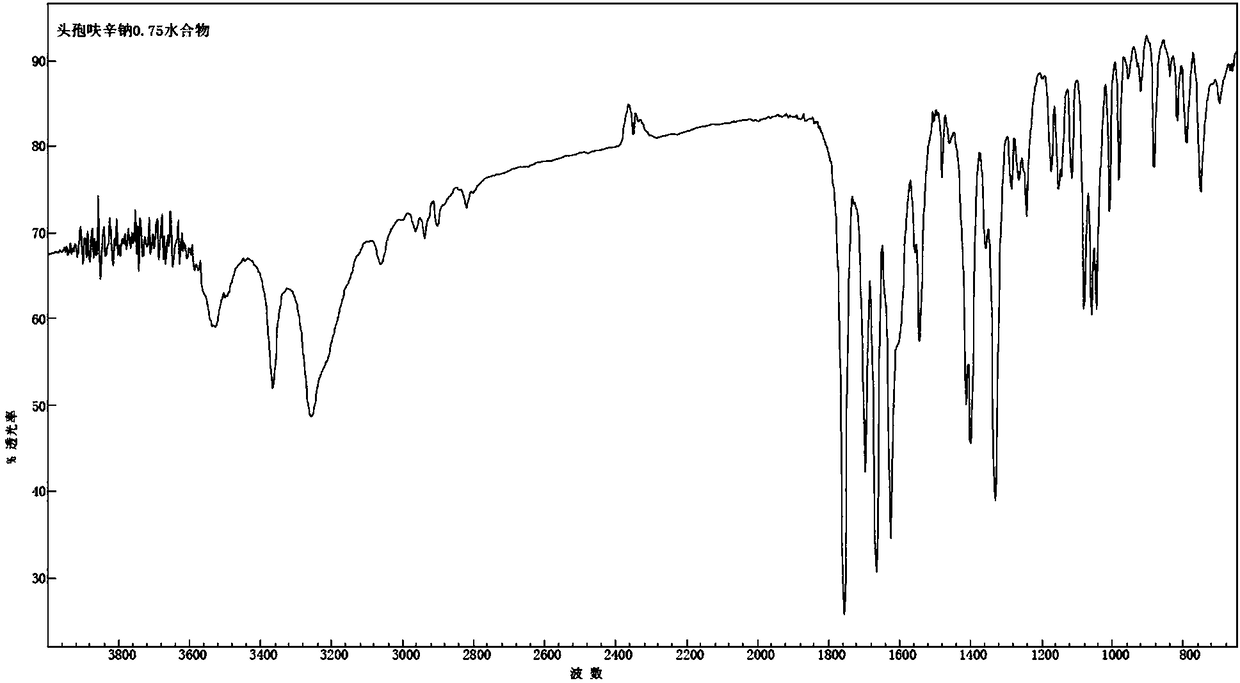
[0036] Fourier transform infrared spectrum at a wavenumber of 3528.3cm -1 ,3366.2cm -1 , 3257.6cm -1 , 1758.1cm -1 , 1699.0cm -1 , 1667.9cm -1 , 1626.9cm -1 , 1402.0cm -1 , 1333.9cm -1 , 1082.6cm -1 , 1061.8cm -1 , 1011.6cm -...
Embodiment 2
[0038] Preparation of Example 2 3 / 4 Cefuroxime Sodium Compound
[0039] (1) At room temperature, add 732g of cefuroxime acid into 4L of dichloromethane, stir, and slowly add sodium lactate (254g) methanol (317ml) solution to react until the pH value is 6.7;
[0040] (2) Add 4L of acetone for elution and crystallization, filter, wash with ethanol, and vacuum-dry at 45°C for 40min to obtain 781g of cefuroxime sodium 3 / 4 water compound.
[0041] Test results:
[0042] The X-ray diffraction pattern has characteristic diffraction peaks at the diffraction angles 2θ of 10.10°, 11.33°, 13.37°, 19.12°, and 19.28°, and the relative diffraction intensities of the diffraction angles are 100, 21.38, 36.47, 96.14, 92.75, and 11.09;
[0043] Fourier transform infrared spectrum at a wavenumber of 3527.7cm -1 , 3366.4cm -1 , 3258.1cm -1 , 1759.5cm -1 , 1698.6cm -1 , 1667.4cm -1 , 1626.4cm -1 , 1402.2cm -1 , 1333.3cm -1 , 1082.4cm -1 , 1062.0cm -1 , 1012.4cm -1 There are characteri...
Embodiment 3
[0045] The preparation of embodiment 3 3 / 4 water cefuroxime sodium compound
[0046] (1) At room temperature, add 606g of cefuroxime acid into 4L of n-propanol, stir, and slowly add sodium lactate (231g) methanol (385ml) solution to react until the pH value is 6.5;
[0047] (2) Add 3L of ethanol for elution and crystallization, filter, wash with ethanol, and vacuum-dry at 45° C. for 40 minutes to obtain 584 g of cefuroxime sodium 3 / 4 water compound.
[0048] Test results:
[0049] The X-ray diffraction pattern has characteristic diffraction peaks at the diffraction angles 2θ of 10.12°, 11.35°, 13.39°, 19.09°, and 19.30°, and the relative diffraction intensities of the diffraction angles are 100, 21.38, 36.47, 96.14, 92.75, and 11.09;
[0050] Fourier transform infrared spectrum at a wavenumber of 3527.0cm -1 , 3366.1cm -1 , 3258.3cm -1 , 1759.9cm -1 , 1698.2cm -1 , 1667.0cm -1 , 1626.1cm -1 , 1402.5cm -1 , 1333.7cm -1 , 1082.8cm -1 , 1062.2cm -1 , 1012.3cm -1 Ther...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com