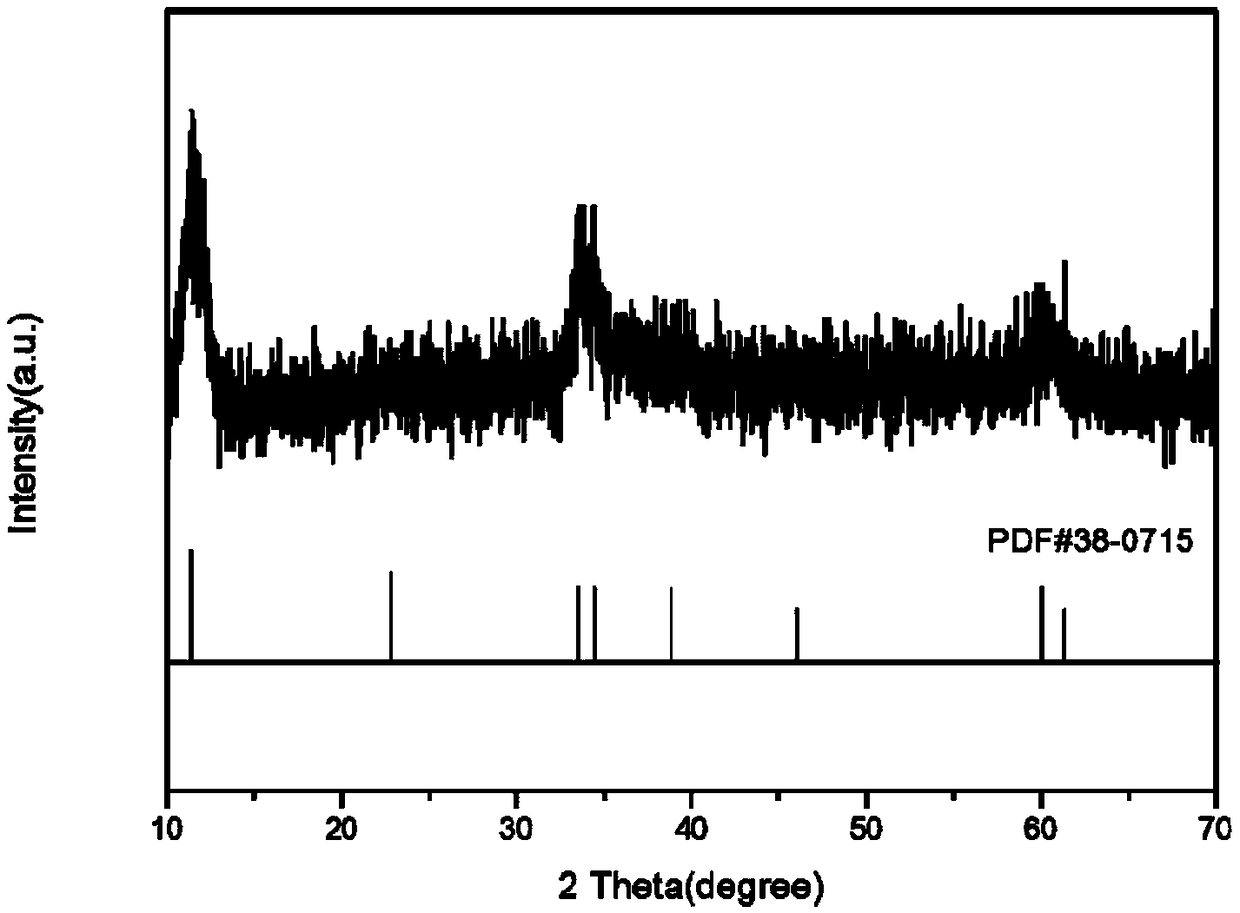
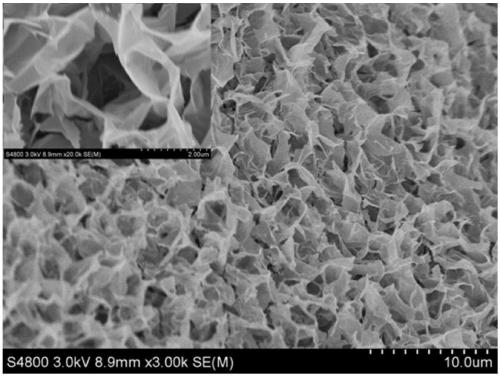
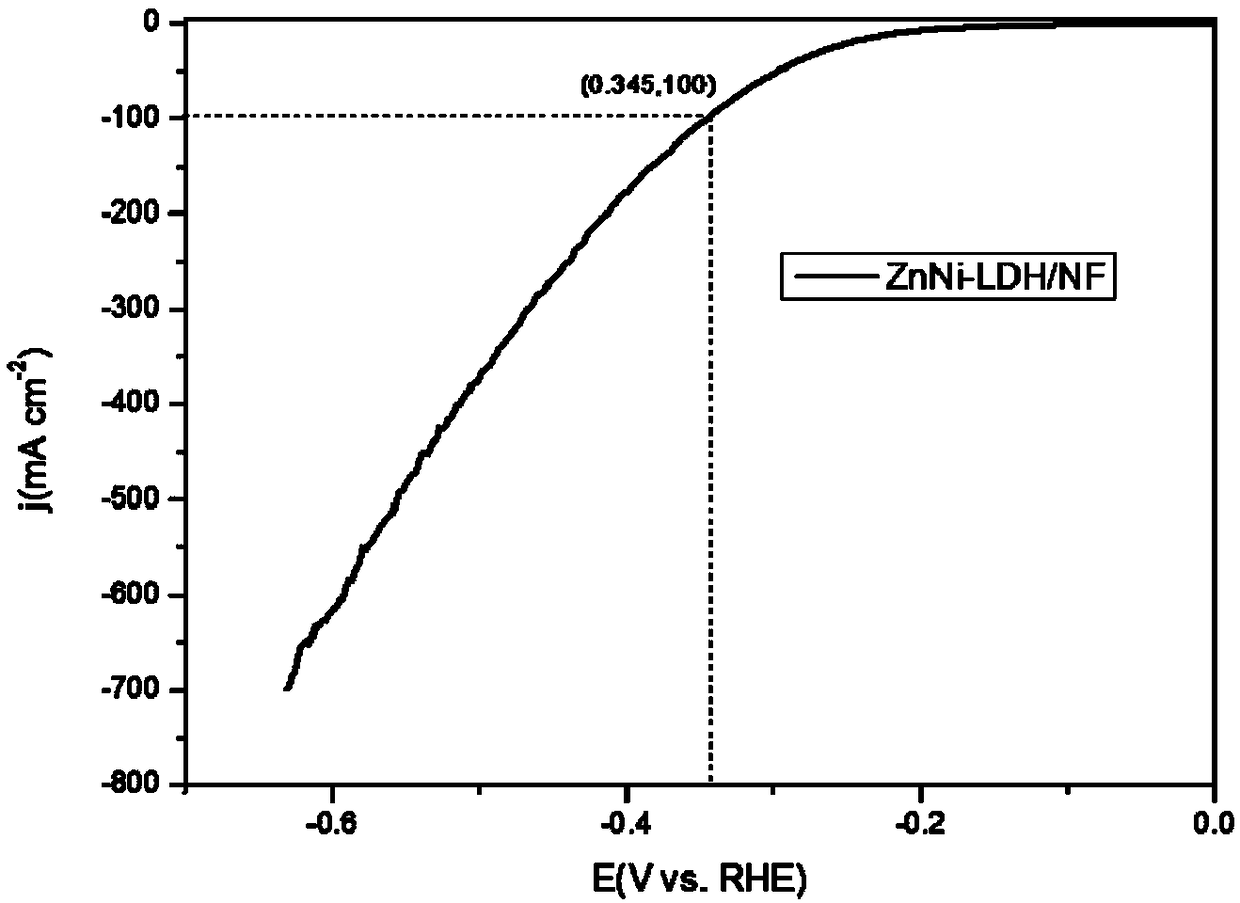
Preparation method of network-structure zinc nickel double-metal hydroxide double-function electrocatalyst
A technology of hydroxide and network structure, which is applied in the field of electrolysis of water, can solve the problems of severe Ni-LDH morphology stacking, uneven distribution of composite product morphology, and large influence of reaction product morphology, achieving excellent electrochemical performance, Simple synthetic route, enhanced electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Immerse the nickel foam in pure acetone solution for ultrasonic cleaning for 10 minutes, then immerse the nickel foam in 2mol / L hydrochloric acid for ultrasonic cleaning for 10 minutes, and finally wash it with ethanol and deionized water alternately for 3 times, and vacuum at 25 °C After drying for 10h, obtain the foamed nickel after processing;
[0028] (2) Weigh NiSO 4 ·7H 2 O, Zn(CH3COO)2·2H 2 O, C 9 h 7 NO and Co(NH 2 ) 2 At the same time, it was added to the ethylene glycol solution to dissolve for 5 minutes, and then a certain volume of deionized water was added. The volume ratio of ethylene glycol solution and deionized water was kept at 4:1 and the total volume of the solution was kept at 60mL. Simultaneous control of NiSO 4 ·7H 2 O, Zn(CH3COO)2·2H 2 O, C 9 h 7 NO and Co(NH 2 ) 2 The molar ratio is 1:0.5:3:10, at this moment the concentration of nickel source is 0.0025mol / L, the concentration of zinc source is 0.0125mol / L, C 9 h 7 The concen...
Embodiment 2
[0032](1) Immerse the nickel foam in pure acetone solution for ultrasonic cleaning for 5 minutes, then immerse the nickel foam in 3mol / L hydrochloric acid for ultrasonic cleaning for 15 minutes, and finally wash it with ethanol and deionized water alternately for 4 times, and vacuum at 25 °C After drying for 12h, obtain the foamed nickel after processing;
[0033] (2) Weigh NiSO 4 ·7H 2 O, Zn(CH3COO)2·2H 2 O, C 9 h 7 NO and Co(NH 2 ) 2 At the same time, it was added to the ethylene glycol solution to dissolve for 10 minutes, and then a certain volume of deionized water was added. The volume ratio of ethylene glycol solution and deionized water was kept at 2:1 and the total volume of the solution was kept at 60mL. Simultaneous control of NiSO 4 ·7H 2 O, Zn(CH3COO)2·2H 2 O, C 9 h 7 NO and Co(NH 2 ) 2 The molar ratio is 2:1:6:15, at this moment the concentration of nickel source is 0.005mol / L, the concentration of zinc source is 0.0025mol / L, C 9 h 7 The concentrat...
Embodiment 3
[0037] (1) Sonically clean the nickel foam in pure acetone solution for 10 minutes, then immerse the nickel foam in 3mol / L hydrochloric acid for 15 minutes, and finally wash it with ethanol and deionized water alternately for 4 times, vacuum at 25°C After drying for 12h, obtain the foamed nickel after processing;
[0038] 2) Weigh NiSO 4 ·7H 2 O, Zn(CH3COO)2·2H 2 O, C 9 h 7 NO and Co(NH 2 ) 2 At the same time, it was added to the ethylene glycol solution to dissolve for 15 minutes, and then a certain volume of deionized water was added. The volume ratio of ethylene glycol solution and deionized water was kept at 4:3 and the total volume of the solution was kept at 60mL. Simultaneously control NiCl 2 ·6H 2 O:Zn(CH3COO)2:Co(NH 2 ) 2 The molar ratio is 3:1.5:9:20, the concentration of nickel source is 0.0075mol / L at this moment, the concentration of zinc source is 0.00375mol / L, C 9 h 7 The concentration of NO was 0.0225 mol / L, the concentration of urea was 0.005 mol / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com