A kind of bipyridine derivative and its synthesis method and application
A synthesis method and derivative technology, applied in the field of fluorescent probes, can solve the problem of inability to distinguish and identify immune responses or two simultaneously participating in immune responses, unable to ensure that fluorescent groups link an antigen and an antibody, affecting the specificity of coupled protein immune responses. To improve the detection and tracking accuracy, improve the sensitivity and specificity, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
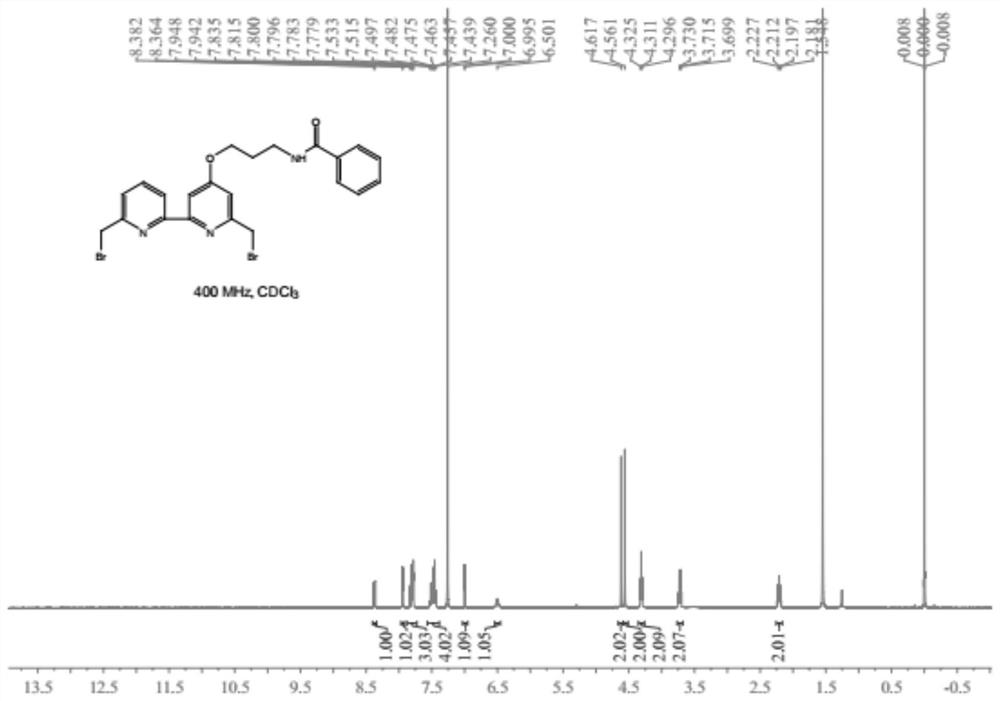
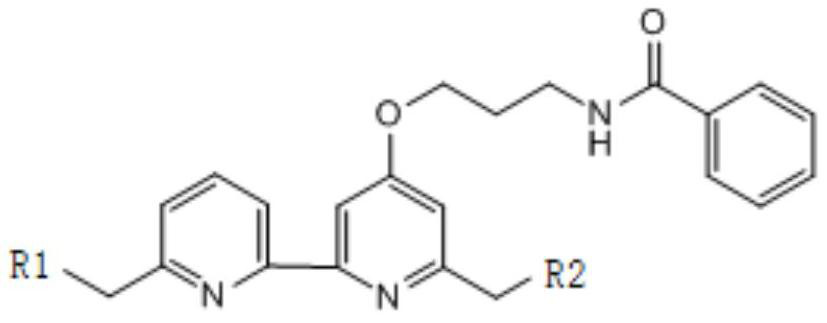
[0053] The synthetic route of compound (1) is as follows:
[0054]
[0055] The synthetic method comprises the following steps:
[0056] A, a pyridine epoxidation of compound (4) is epoxidized with m-CPBA to generate compound (5);
[0057] B, compound (5) is nitrated with sulfuric acid and nitric acid to generate compound (6);
[0058] C, the nitro group on the compound (6) is converted into bromine with acetyl bromide to generate compound (7);
[0059] D, compound (7) is reduced to compound (8) with phosphorus tribromide;
[0060] E, compound (8) is condensed with 3-amino propanol, generates compound (9);
[0061] F, compound (9) reacts with benzoyl chloride to generate compound (10);
[0062] G, compound (10) is oxidized to compound (11) with m-CPBA;
[0063] H, compound (11) reacts with acetic anhydride, reduces N-oxide compound, generates compound (12);
[0064] 1. Compound (12) is hydrolyzed into compound (13);
[0065] J. Bromating compound (13) with phosphorus t...
Embodiment 2
[0067] This embodiment is on the basis of embodiment 1:
[0068] In step A, one pyridine of compound (4) is epoxidized with benzoic acid peroxide.
[0069] The compound that undergoes acylation reaction with compound (9) in step F is benzyl chloroformate.
Embodiment 3
[0071] This embodiment is on the basis of embodiment 1:
[0072] In the step A, one pyridine of compound (4) is epoxidized with ammonium persulfate or the like.
[0073] The compound that undergoes acylation reaction with compound (9) in step F is tert-butyl chloroformate.
[0074] The bromination reagent used in the J step is hydrogen bromide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com