Production process of high-titer tuberculin skin test diagnostic reagent PPD (Purified Protein Derivative)
A production process, tuberculin technology, applied in the field of immunology, can solve the problem of inability to solve the problem of fine bands, the complexity of PPD production process, the technical difficulty and the definition of components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 bacterial culture
[0047] (1) 1 seed of recovery work was inoculated into 5 Roche's egg culture medium, and cultured for 2 weeks. After the bacteria grew into dry, wrinkled and slightly light yellow bacterial film, the growth of the bacterial lawn could be observed at this time: the end of the culture After that, each 1st-generation strain was first suspended with 5ml of sodium chloride 0.9% solution, collected all the bacterial liquid, and sampled for acid-fast staining. Acid-fast staining was positive (bacteria were stained red). Biochemical reaction: Nitrate reduction reaction is positive, resulting in red; urease reaction is positive, showing red; heat-resistant catalase reaction should be negative, no bubbles are generated in 10-20 minutes; polysorbate 80 hydrolysis reaction does not change color. Each medium tube was resuspended with 2ml of physiological saline to prepare a bacterial solution, and a total of 10ml (1st generation) was collected. The bac...
Embodiment 3
[0061] Example 3 Precipitation
[0062] A 40% trichloroacetic acid solution was prepared, added to the harvest solution at a ratio of 1:10, with a final concentration of 4%, and allowed to stand at room temperature for 2 hours. After shaking well, centrifuge at 4°C and 8000rpm for 20min. The precipitate was collected and weighed 450 g.
[0063]Precipitate 450g is dissolved with 3150ml of 0.9% sodium chloride solution, adjusts the pH value with 1M sodium hydroxide, adds ammonium sulfate according to the amount of precipitation dissolution, makes its concentration reach 60% saturation. Add 390g×3.15L=1228.5g of ammonium sulfate, let stand at 2-8°C overnight (16 hours), centrifuge at 8000rpm for 20min at 4°C, and collect 365g of precipitate. Add 2555ml of 0.9% sodium chloride solution to dissolve the precipitate. Add 40% trichloroacetic acid to a final concentration of 4%, precipitate protein, centrifuge at 8000 rpm, collect the precipitate, and weigh 310 g. The precipitation...
Embodiment 5
[0066] Embodiment 5 sterile filtration
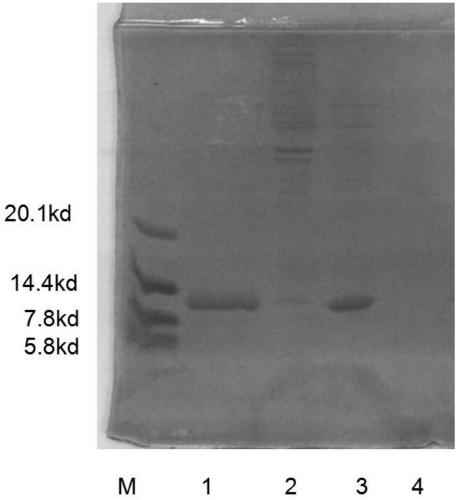
[0067] Filter and clarify the dialysate through a 1.2μm~0.45μm microporous membrane, take a 10ml sample after filtration to determine the microbial limit, and then sterilize and filter through a 0.45μm~0.22μm filter membrane to obtain the stock solution. Harvest a total of 1800ml, take a sample for testing, and test items For protein content, polysaccharide content, nucleic acid content, sterility test. The sterilization and filtration time should be controlled within 4 hours. The test result shows that the protein recovery rate is 60%, and the recovery rate%=(liquid stock solution ml×liquid stock solution protein content mg / ml) / (filtrate volume ml×filtrate protein content mg / ml), wherein the protein content is 1.75 mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com