Fluorescent quantitative PCR kit for simultaneous detection of hepatitis B Virus, hepatitis C Virus and human immunodeficiency virus Type 1
A technology of simultaneous detection and kits, applied in the direction of microorganism-based methods, measurement/testing of microorganisms, microorganisms, etc., can solve the problems of unsuitable screening of blood donors and high cost, and achieve short detection time, low detection cost, The effect of high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The preparation of embodiment 1 kit
[0079] According to the genome sequences of HBV, HCV and HIV-1 viruses, the sequences of detection primers and probes (SEQ ID NO.1~SEQ ID NO.12) were designed respectively, and the detection primers and probes of HBV, HCV and HIV-1 were synthesized respectively .
[0080] The primer probe sequences for detecting HBV are shown in SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3:
[0081] SEQ ID NO.1: Upstream primer: 5'-TGTCTGCGGCGTTTTATCA-3'
[0082] SEQ ID NO.2: Downstream primer: 5'-TAGTCCAGAAGAACCAACAAGAA-3'
[0083] SEQ ID NO.3: Taqman probe: 5'-ATGAGGCATAGCAGCAGGAT-3', the 5' fluorescent group of the probe is labeled with FAM, and the 3' quencher group is labeled with MGB.
[0084] The primer probe sequences for detecting HCV are shown in SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3:
[0085] SEQ ID NO.4: Upstream primer: 5'-TGGCGTTAGTATGAGTGTCGT-3'
[0086] SEQ ID NO.5: Downstream primer: 5'-GACCACTATGGCTCTCCCG-3'
[0087] SEQ ID NO...
Embodiment 2
[0098] The detection sensitivity analysis of embodiment 2 kit
[0099] Prepare a PCR reaction system in the reagent preparation room as follows, PCR reaction solution: 16 μl×n, enzyme system: 2 μl×n, primer-probe mixture: 2 μl×n. Dispense the PCR reaction liquid into PCR reaction tubes according to 20 μl / tube, and move the reaction tube containing the PCR reaction liquid to the sample processing area. Use a suction nozzle with a filter element to take 5 μl of the supernatant of the processed sample, negative control, and positive control, respectively, and add them to the PCR reaction tubes equipped with the reaction system. Close the tube cap, centrifuge for a few seconds and move to the amplification detection area. Put the PCR reaction tube into a fluorescent PCR amplification instrument for amplification detection, and the reaction program of PCR amplification is shown in Table 3.
[0100] The results of PCR amplification were analyzed according to the following criteria: ...
Embodiment 3
[0112] The detection precision analysis of embodiment 3 kits
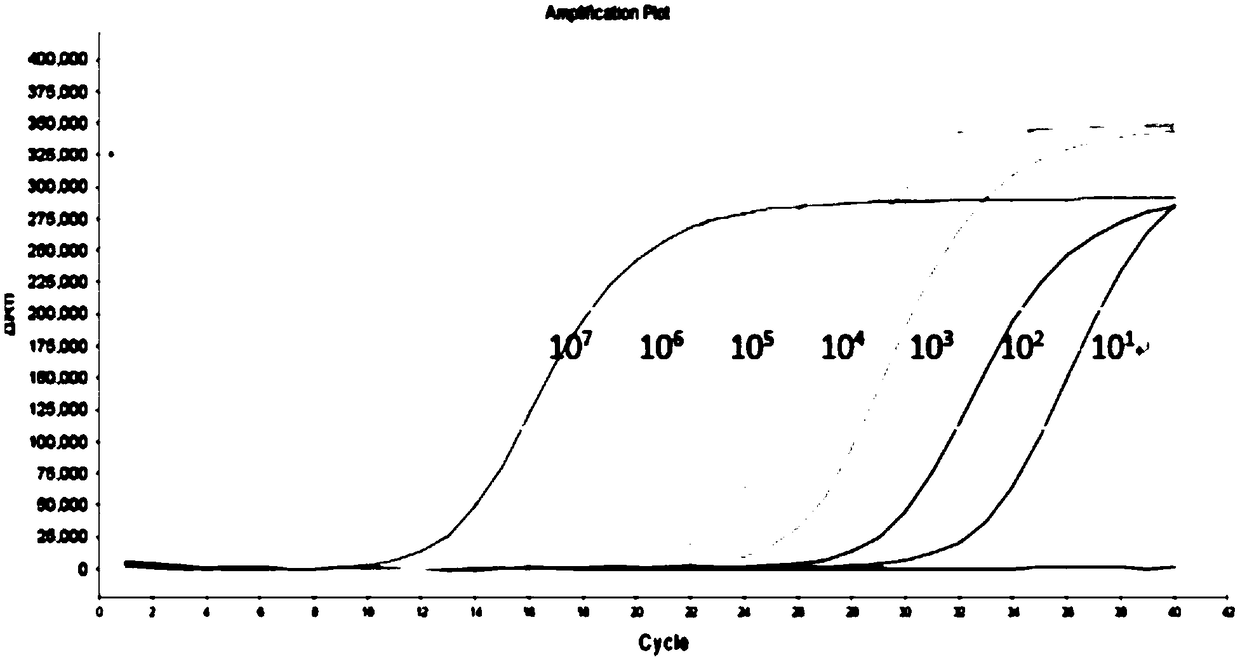
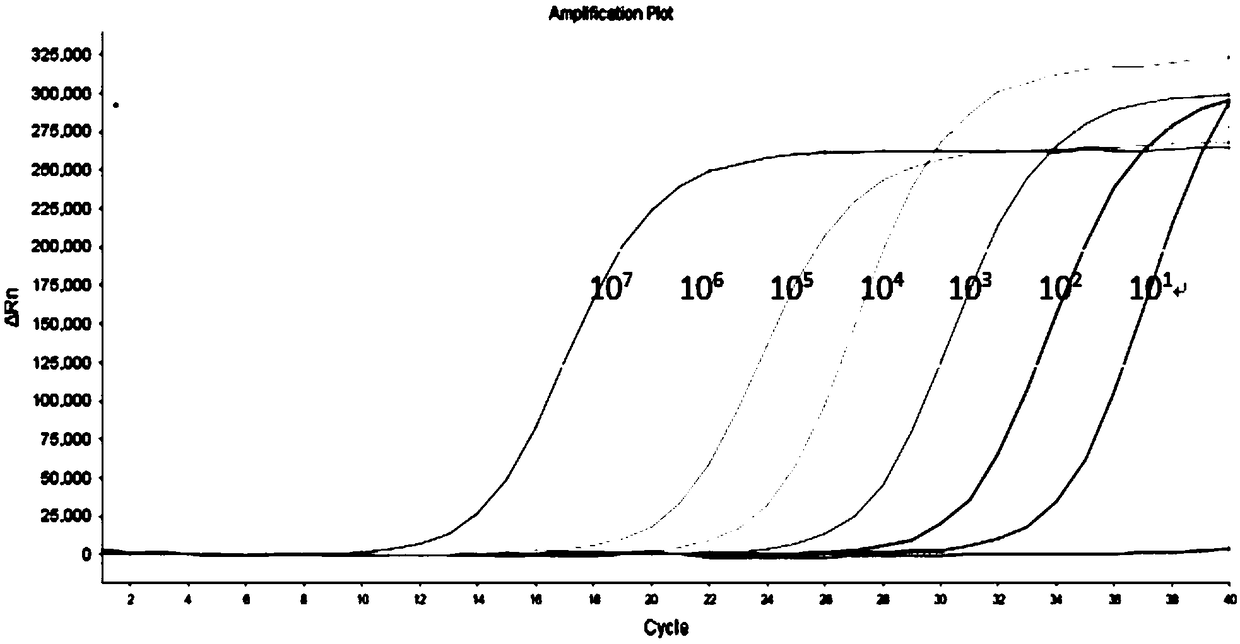
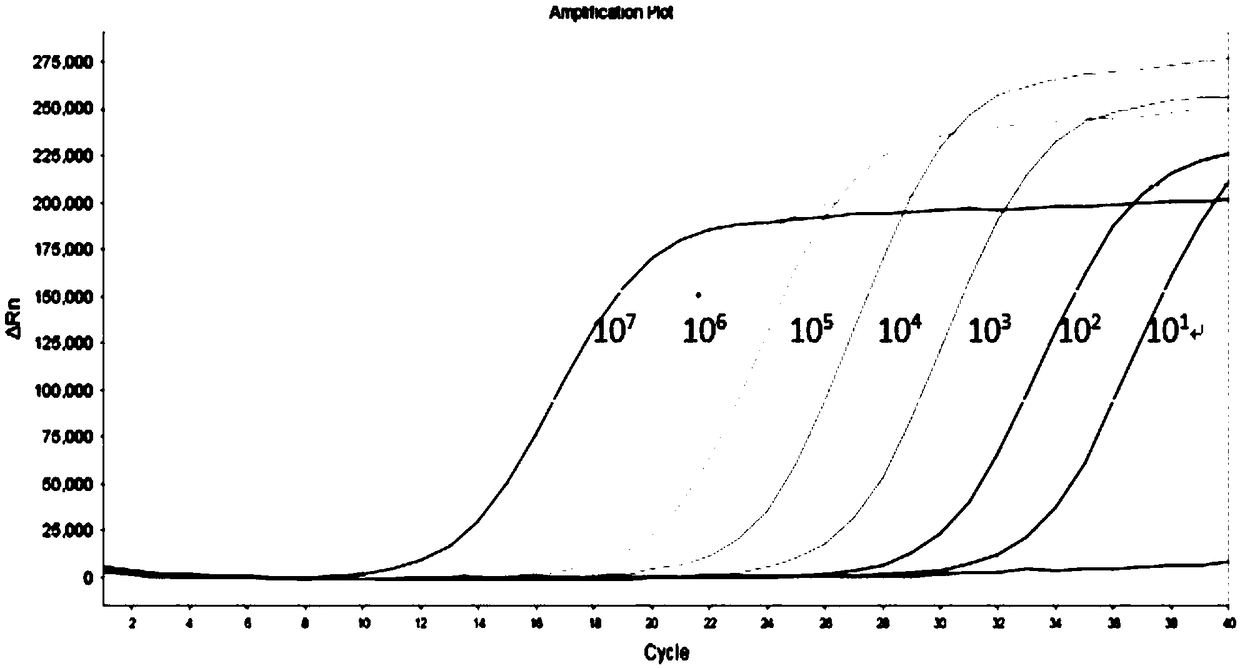
[0113] Adopt the method described in embodiment 2, utilize the fluorescent quantitative PCR kit that embodiment 1 provides to detect HBV, HCV and HIV-1 synchronously, to HBV positive control clone plasmid (1 * 10 6 copies / μl, 1×10 4 copies / μl) as the sample to be tested for HBV, for HCV positive control virus-like particles (1×10 4 copies / μl, 1×10 2 copies / μl) as HCV samples to be tested, for HIV-1 positive control virus samples (1×10 4 copies / μl, 1×10 1 copies / μl) as the sample to be tested for HIV-1. Use a quality-tested fluorescent quantitative PCR kit for the simultaneous detection of HBV, HCV, and HIV-1 for detection, each of which is repeated 8 times. Detection was performed on an ABI7500 instrument. The results showed that the coefficients of variation of the detection Ct values of different nucleic acid concentrations were all Figure 5 , HCV precision test see Figure 6 , HIV-1 precision test se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com