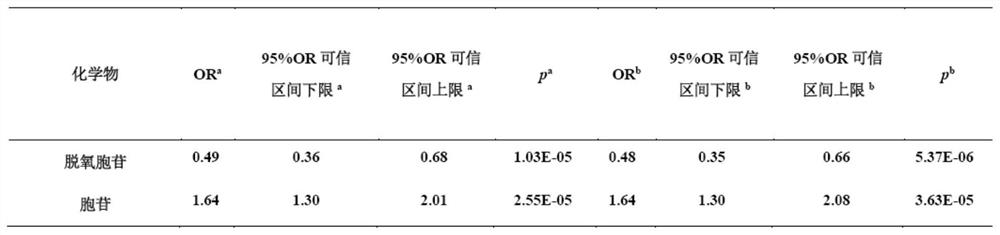
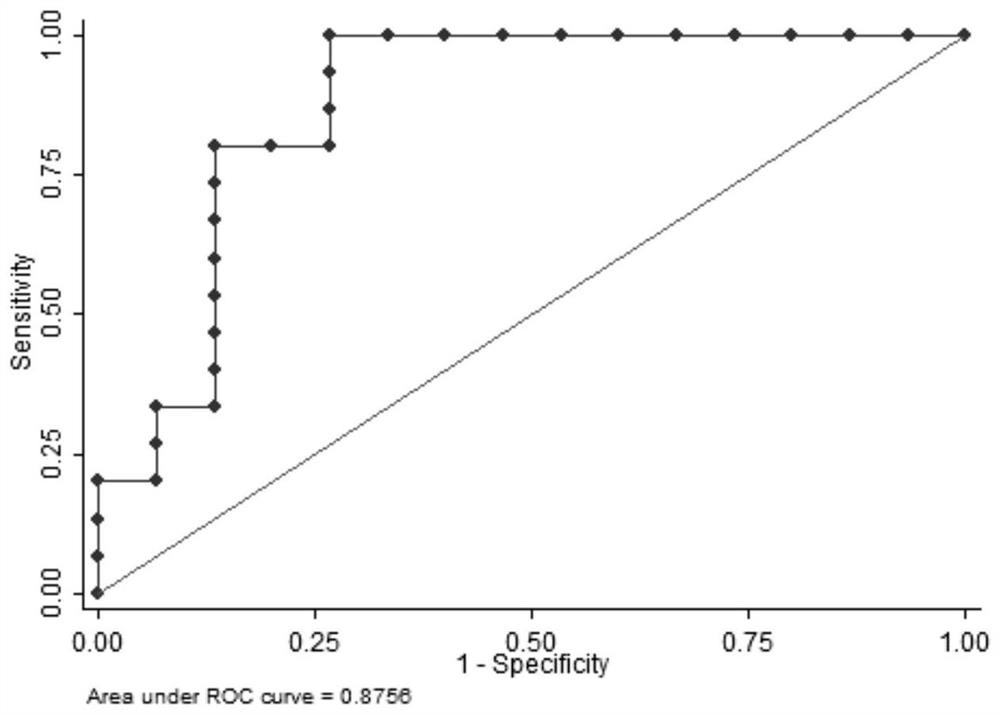
Detection of seminal plasma deoxycytidine and cytidine as diagnostic markers for idiopathic male infertility and its application
A technology for male infertility and deoxycytidine, which is applied in the fields of analytical chemistry and clinical medicine, can solve the problems of complex experimental steps and low sensitivity, and achieve the effects of improved sensitivity and specificity, high sensitivity and specificity, and easy detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 Study object selection and grouping according to embodiment
[0106] This part of the study objects from Nanjing Medical University Hospital of first diagnosis of idiopathic cases of male infertility and reproductive health controls. Research and informed consent were approved by the ethics committee of the Medical University in Nanjing, meets the requirements of relevant laws and regulations. Cases and controls in the understanding of the content of signed informed consent. All subjects underwent a complete physical examination and completed a basic information including personal, lifestyle, occupational and environmental exposures, genetic risk factor questionnaire, sexual function and reproductive function, disease history and physical activity. The first stage 148 to meet the requirements included idiopathic male infertility cases and 148 healthy controls; 15 meet the requirements of the second stage idiopathic male infertility patients and 15 healthy controls...
Embodiment 2
[0138] Example 2 UPLC-MS metabolic sygental male infertility deoxytidine and cytosine screening
[0139] Pre-sample processing
[0140] 1.1. Take 10 μl of the semicake, add 10 μl of internal standard A, add 10 μl of internal standard B, add 10 μl of internal standard C, add 40 μl of methanol (reagent A), vortex 30s, precipitation protein.
[0141] 1.2. The centrifuge is centrifuged at 4 ° C for 15 min, and the supernatant was transferred to a 1.5 mL of the inlet EP tube, and the supernatant was concentrated in the centrifugal concentration dryer at room temperature.
[0142] 1.3. With 10 μl of ultra-pure water (reagent D), it is prepared.
[0143] 2. Instrument detection
[0144] 2.. Analysis Instrument: UPLC Ultimate 3000System (Dionex) High Performance Liquid Chromatograph; Q-EXACTIVE High Differentiation Mass Spectrometer.
[0145] 2.2. Liquid phase conditions:
[0146] 2.2.1 Liquid chromatography column is Hypersil Gold C18 column (100 mm × 2.1mm, particle size 1.9 μm, thermo ...
Embodiment 3
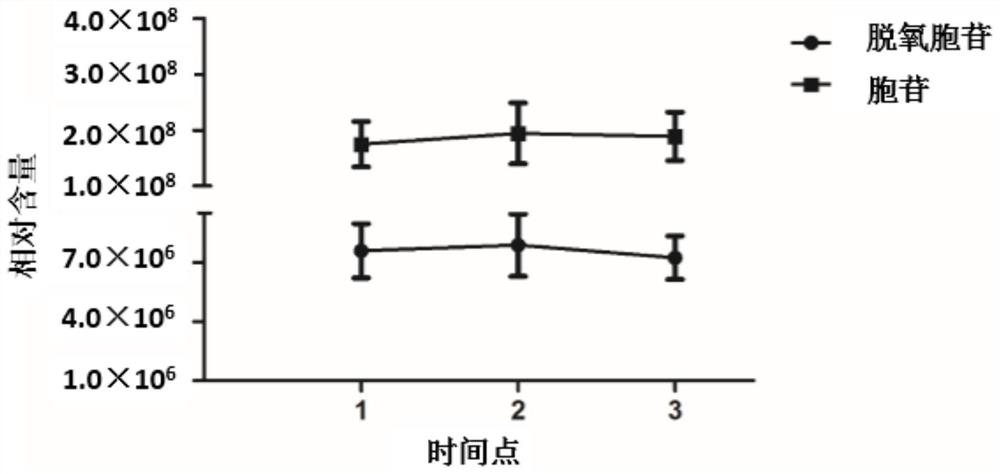
[0159] Example 3 Stability analysis of sequetal deoxytidine and cytanoside
[0160] The stability of the sequetal deoxycytidine and the cytosine level was evaluated using the method of Example 2 (the interval time was 2 weeks). The results show that the levels of deoxyphine and cytosine assay in the semers are stable ( figure 2 ), Have the characteristics of diagnostic / monitoring markers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com