Simultaneous Quantitative Determination of 9 Blood Components in Suanzaoren Water Extract
A technology for quantitative determination and blood components, applied in measuring devices, material separation, and analysis of materials, can solve the problems of unfavorable pharmacokinetic research of traditional Chinese medicine, long analysis time, low analytical sensitivity, etc., and achieve comprehensive pharmacokinetic research , Shorten the analysis time and improve the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
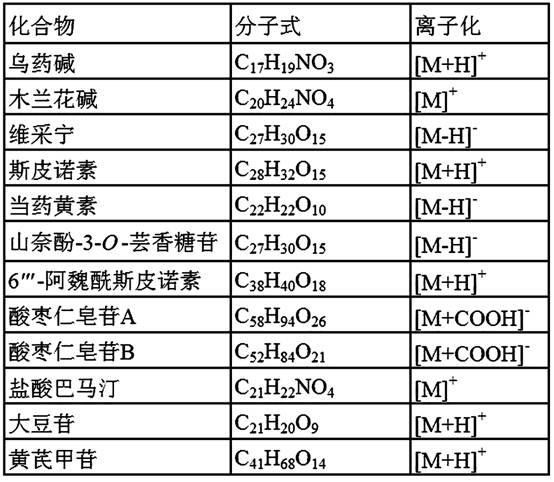
[0027] Example 1: A method for the simultaneous quantitative determination of 9 blood components in the water extract of Suanzaoren, using the UHPLC-QExactive Orbitrap HR / MS method to quantitatively determine 9 active ingredients in the water extract of Suanzaoren: black medicine Alkaline, magnolialine, weixining, spinosin, flavin, kaempferol-3- O -Rutinoside, 6 ''' - Feruloyl spinosin, jujube saponin A and jujube saponin B; the specific steps are as follows:
[0028] (1) Preparation of a single stock solution of a reference substance: Accurately weigh the reference substances respectively, add the initial mobile phase of the chromatography analyzed by UHPLC-Q ExactiveOrbitrap HR / MS, and prepare 2.032 mg / mL of aconitine and 1.130 mg / mL of magnolialine The stock solution of single reference substance was prepared by adding methanol to Weicaining 2.184 mg / mL, spinosin 2.016 mg / mL, flavin 1.972 mg / mL, kaempferol-3- O - Rutinoside 2.058 mg / mL, 6 ''' - A single reference substan...
Embodiment 2
[0038] Embodiment 2: methodological investigation
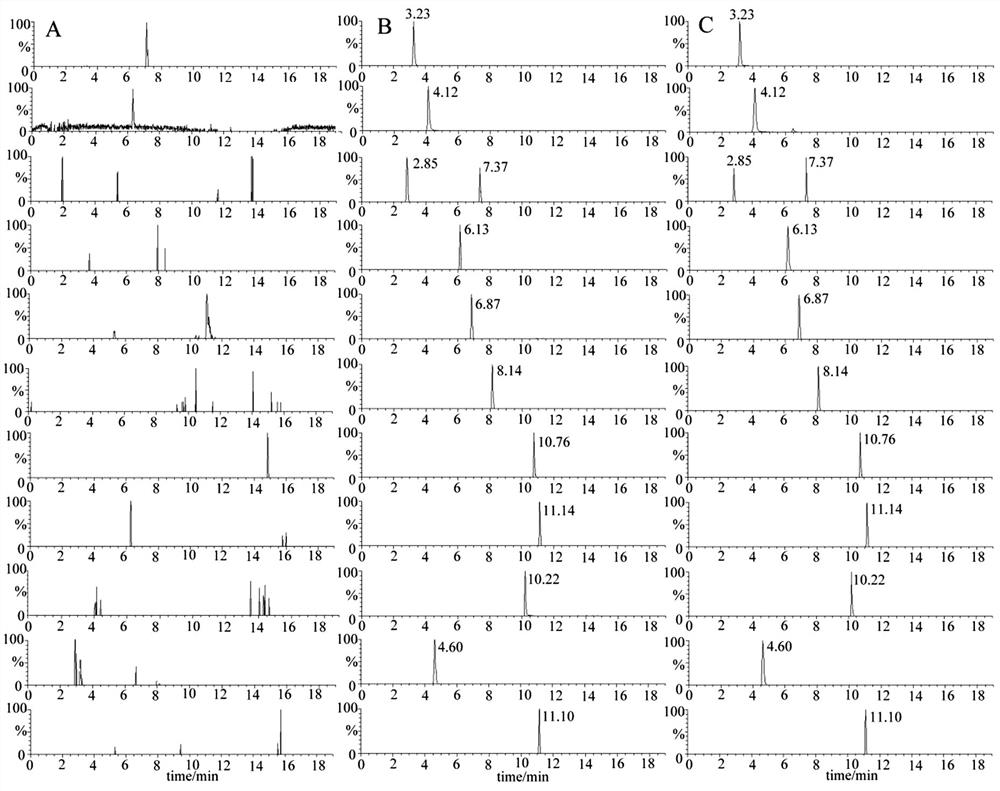
[0039] 1. Specificity test: the blank plasma, blank plasma plus working solution, and drug-containing plasma from 6 rats from different sources were processed and analyzed according to the conditions in Example 1. Compare the chromatograms of the three, and observe whether there is interference in the blank plasma at the peak positions of the components to be tested and the internal standard. The result is as figure 1 As shown, the retention times of the components to be tested and the internal standard were respectively: 3.23 min for asteroidine, 4.12 min for magnolialine, 2.85 min for weicaining, 6.13 min for spinosin, 6.87 min for flavin, and 6.87 min for kaempferol-3 - O - Rutinoside 7.37 min, 6 ''' - Feruloyl spinosin 8.14 min, jujuboside A 10.76 min, juboside B 11.14 min, palmatine hydrochloride 10.22 min, daidzin 4.60 min, astragaloside 4 11.10 min. There was no interference in the blank plasma corresponding to th...
Embodiment 3
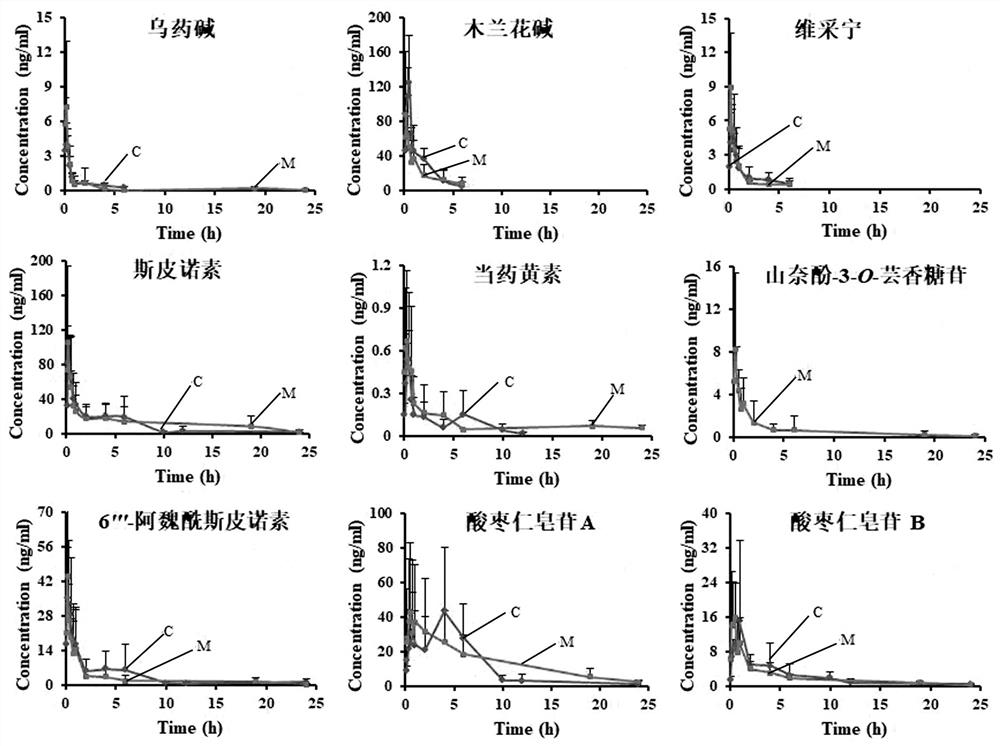
[0052] Example 3: Pharmacokinetic determination of 9 components in rat plasma after oral administration of jujube seed aqueous extract in normal and PCPA insomnia model rats
[0053] (1) Preparation of Suanzaoren water extract: take about 500 g of Suanzaoren decoction pieces, crush (60% pass through No. 1 Pharmacopoeia sieve), accurately weigh, add 10 times the amount of water, soak for 30 min, heat to boiling, and reflux extraction 2 h, filter, add 8 times the amount of water to the filter residue, heat and reflux for extraction for 2 h, filter, and combine the filtrates; concentrate under reduced pressure to 0.5 g / mL (crude drug amount), freeze-dry, and dissolve in water when used.
[0054] (2) Animal experiments and collection of plasma samples: healthy male SD rats, weighing 280±20 g, provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The rats were randomly divided into two groups, the normal group and the model group, with 6 rats in each group. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com