Method for simultaneously and quantitatively detecting two components entering blood in trichosanthes kirilowii maxim, allium macrostemon and pinellia ternate and application
A quantitative detection method, the technology of Gualou Xiebai, is applied in the direction of measuring devices, material separation, and analysis of materials, so as to shorten the analysis time and improve the detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] Preparation of internal standard solution: Weigh 5.00 mg of zeatin into a 10 mL volumetric flask, add methanol solution to the volume to obtain a 0.5 mg / mL internal standard stock solution, and dilute to obtain a 4 μg / mL internal standard solution for later use;
[0045]Preparation of plasma samples to be tested: Precisely draw 50 μL of rat plasma samples into 1.5 mL of EP, precisely add 10 μL of the above internal standard solution, vortex for 1 min to mix evenly, then add 140 μL of acetonitrile solution, vortex for 5 min, and mix thoroughly Evenly, centrifuge at 12000g for 10min, and the supernatant is the plasma sample to be tested;
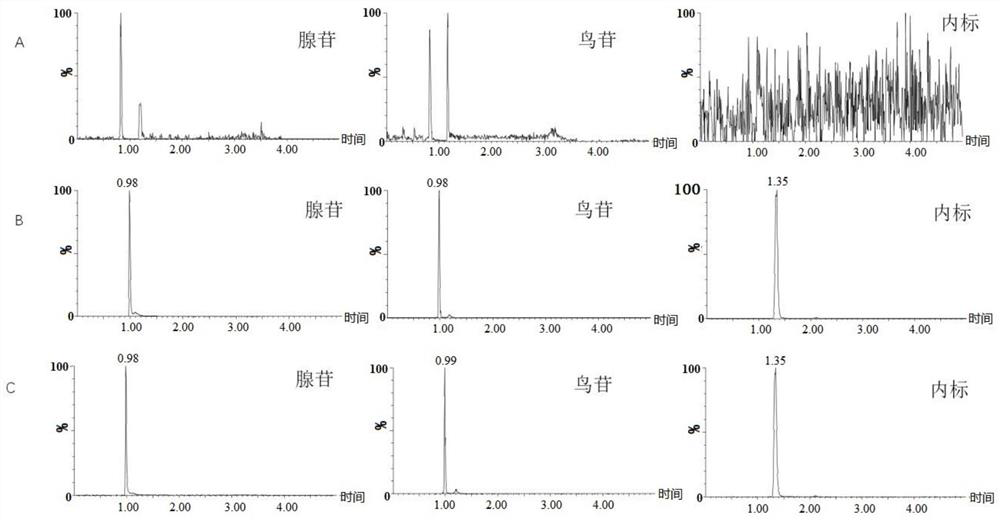
[0046] Using UPLC-MS / MS to simultaneously detect the contents of guanosine and adenosine in the plasma samples to be tested;
[0047] Among them, mass spectrometry conditions: the ion source is ESI source, the capillary voltage is 3.2kv, the desolvation gas is nitrogen; the temperature of the desolvation gas is 500°C, the flow rate of t...
Embodiment 1
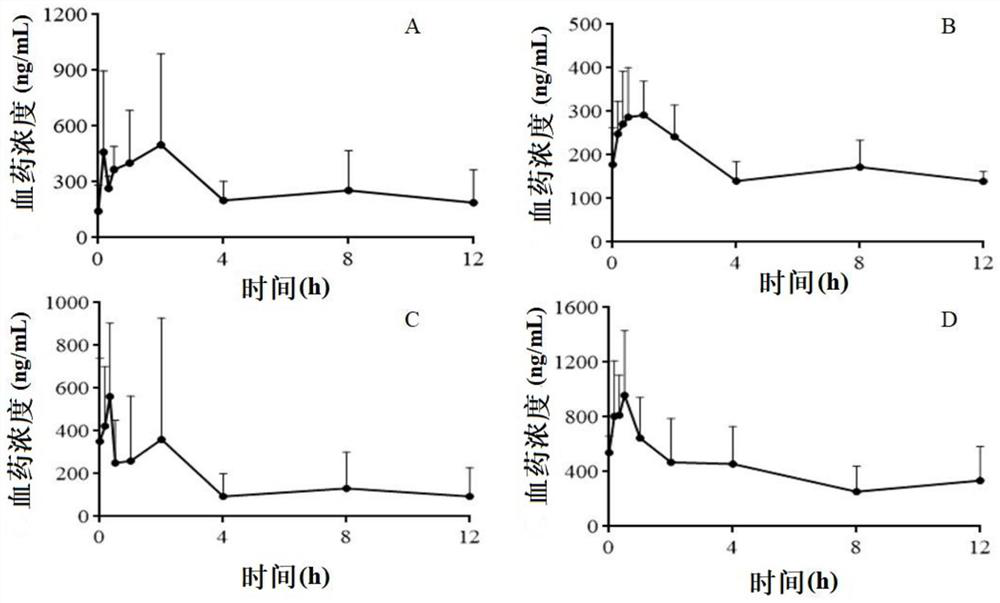
[0064] Example 1 Study on the Determination Method of Gualou Xiebai Banxia Drug Entering Blood Components
[0065] 1. Solution Preparation
[0066] 1.1 Preparation of mixed reference solution
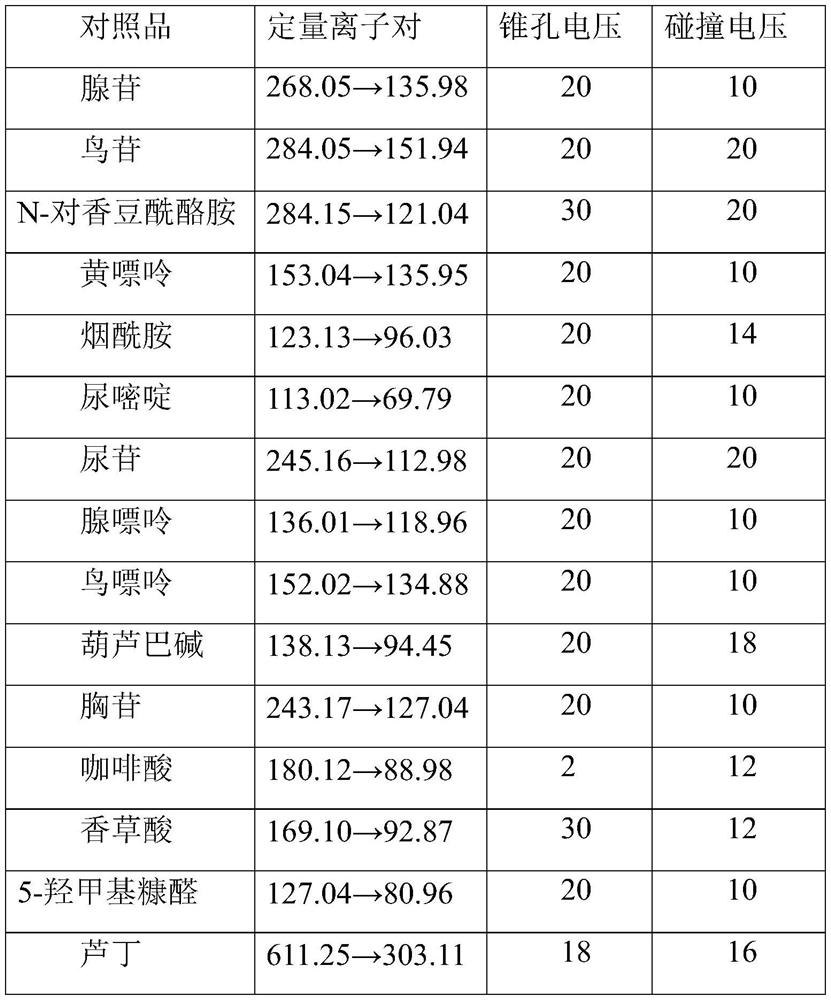
[0067] Precisely weigh the reference substances trigonelline, uracil, thymidine, adenine, caffeic acid, uridine, guanine, guanosine, xanthine, adenosine, vanillic acid, 5-hydroxymethylfurfural, nicotinamide, Ding, quercetin, luteolin, protocatechuic acid, homoharringtonine, schiaftoside, cucurbitacin B, N-p-coumaroyl tyramide and ferulic acid 5.00mg each, placed in 10mL In the volumetric flask, add methanol to make the volume up to the mark, so that the final concentration is 0.5 mg / mL of each reference substance stock solution;
[0068] Then accurately measure each reference substance stock solution respectively, mix and dilute with methanol to prepare a mixed reference substance solution; the concentration of each reference substance in the mixed reference substance solution is as f...
Embodiment 2
[0099] Example 2 Methodological verification of the assay method for blood component content of Gualou Xiebai Pinellia jelly
[0100] Limit of detection and limit of quantitation (confirmation of prototype components in blood)
[0101] According to the signal-to-noise ratio of each reference substance in the mixed reference substance solution, calculate the required dilution factor, and properly dilute each reference substance stock solution to obtain the concentration of each reference substance in the mixed reference substance solution, and then follow the detection conditions under 2.1 Detect, determine the limit of quantification with the signal-to-noise ratio (S / N) greater than 10, determine the limit of detection with the signal-to-noise ratio (S / N) greater than 3, the detection limit and the limit of quantification of 22 kinds of components are indicated.
[0102] Table 5 Limits of quantification and detection of 22 components in rat plasma
[0103]
[0104]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com