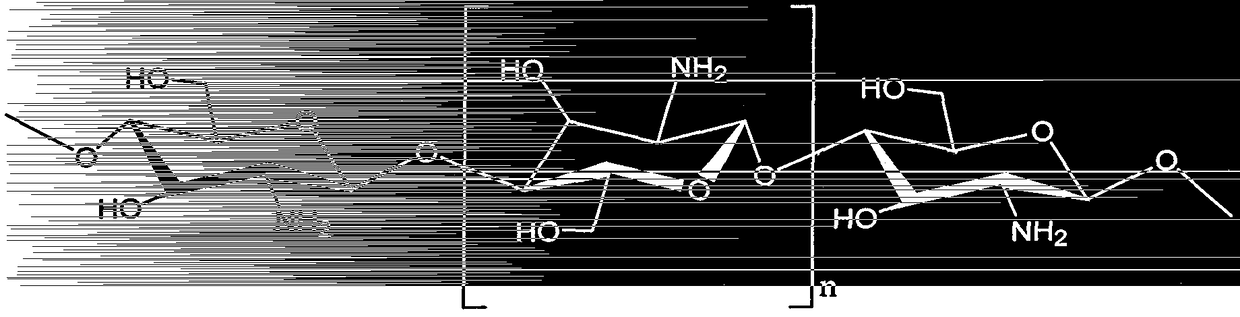
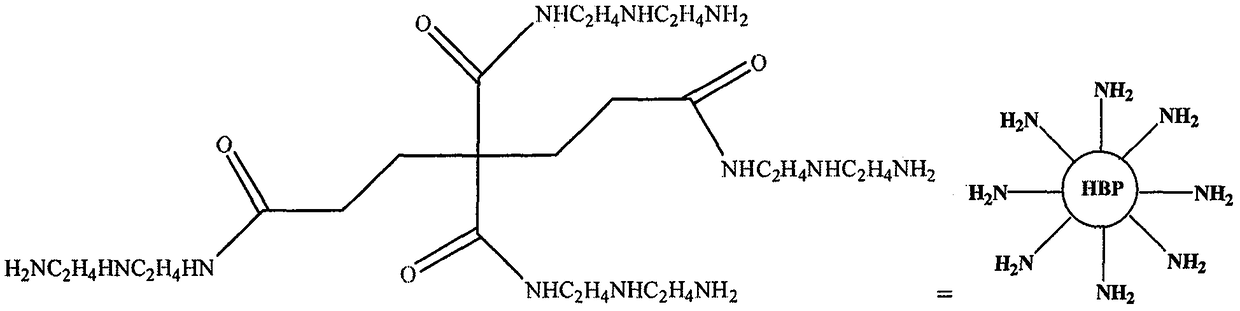
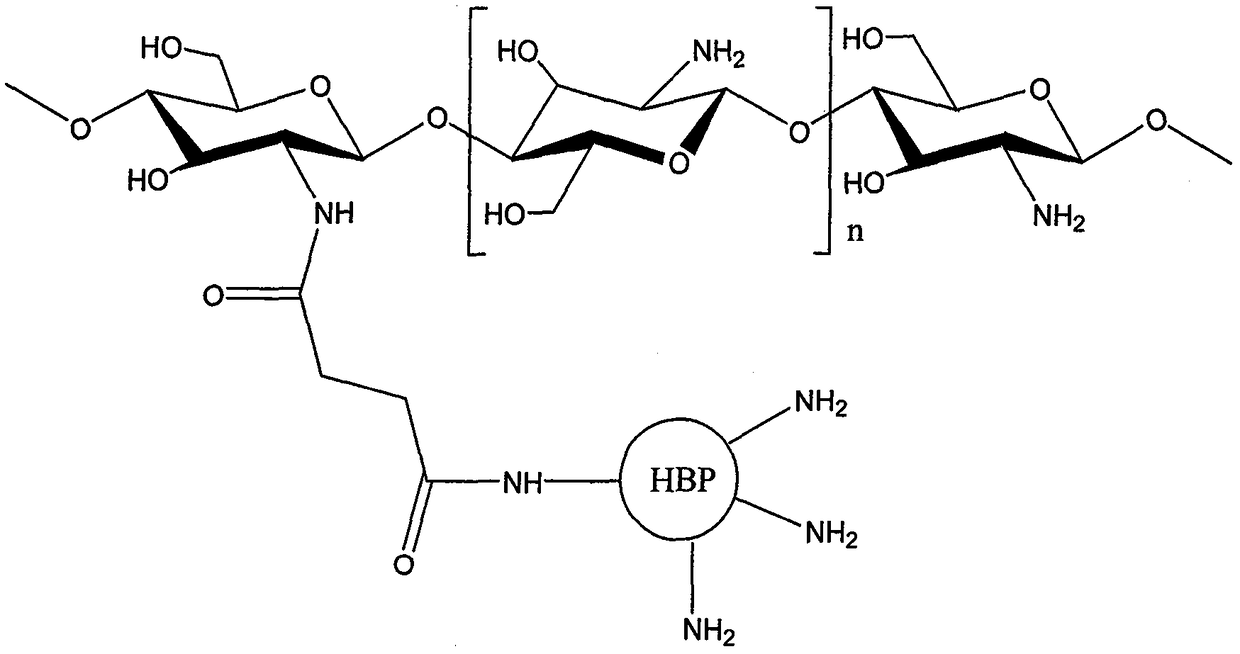
Preparation method of amino-terminated hyper-branched polymer grafted chitosan microsphere formaldehyde adsorbent
A technology of amino-terminated hyperbranched and hyperbranched polymers, applied in alkali metal compounds, chemical instruments and methods, separation methods, etc., can solve the problems that limit the wide application of chitosan, chitosan anti-swelling, poor solubility, etc. , to achieve the effect of large enrichment factor, good reproducibility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthetic tetracarboxylic acid ester: 15.2ml of diethyl malonate of 0.1mol, petroleum ether 50ml, tetrabutylammonium bromide 1.5g, K 2 CO 3 2.76g, 0.25mol methyl acrylate was added dropwise. Control the temperature at 64°C-66°C for 4h to obtain a white solid.
[0026] (2) Synthesis of amino-terminated hyperbranched polymers: 13.66 g of tetravalent carboxylate, 40 ml of methanol, 15 mL of diethylenetriamine was added dropwise, reacted at room temperature for 2 h under nitrogen protection, removed methanol under reduced pressure, and continued to heat up to 80 °C under reduced pressure. ℃, reacted for 3 hours, a light yellow viscous product was generated, and the hyperbranched polymer was obtained.
[0027] (3) chitosan grafted succinic anhydride: in the step 2, the acid anhydride compound adopts succinic anhydride, and 1g chitosan is dissolved in 1-allyl-3-methylimidazolium chloride salt ionic liquid earlier , dissolved for 60 min, then added 1.5 g of succinic anh...
Embodiment 2
[0031] (1) synthetic tetravalent carboxylic acid ester: dimethyl malonate 16ml of 0.1mol, ethyl acetate 50ml, benzyltriethylammonium chloride 1.5g, K 2 CO 3 2.83g, 0.25mol butyl acrylate was added dropwise. After reflux for 4 hours, the temperature was controlled between 64°C and 66°C, and a white solid was generated.
[0032] (2) Synthesis of amino-terminated hyperbranched polymers: 15.12 g of tetravalent carboxylate, 50 ml of n-butanol, 15 mL of ethylenediamine was added dropwise, reacted at room temperature for 2 h under nitrogen protection, removed methanol under reduced pressure, and continued to heat up to 80°C, reacted for 3 hours, a light yellow viscous product was generated, and the hyperbranched polymer was obtained.
[0033] (3) chitosan grafted succinic anhydride: in described step 2, acid anhydride compound adopts succinic anhydride, first 1g chitosan is dissolved in 1-butyl-3-methylimidazolium chloride salt ionic liquid, Dissolved for 60 minutes, then added 1....
Embodiment 3
[0037] (1) Synthetic tetracarboxylic acid ester: 14.72ml of diethyl malonate of 0.1mol, 50ml of cyclohexane, 1.5g of tetrabutylammonium chloride, K 2 CO 32.69g, 0.25mol of ethyl acrylate was added dropwise. After reflux for 4 hours, the temperature was controlled between 64°C and 66°C, and a white solid was generated.
[0038] (2) Synthesis of amino-terminated hyperbranched polymers: 14.12 g of tetravalent carboxylate, 40 ml of ethanol, 15 mL of triethylenetetramine was added dropwise, reacted at room temperature for 2 h under nitrogen protection, removed methanol under reduced pressure, and continued to heat up to 80 °C under reduced pressure. ℃, reacted for 3 hours, a light yellow viscous product was generated, and the hyperbranched polymer was obtained.
[0039] (3) Chitosan grafted succinic anhydride: in the step 2, the acid anhydride compound adopts succinic anhydride, and 1g chitosan is dissolved into 1-ethoxycarbonylmethyl-3-methylimidazole acetate Dissolve in the io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com