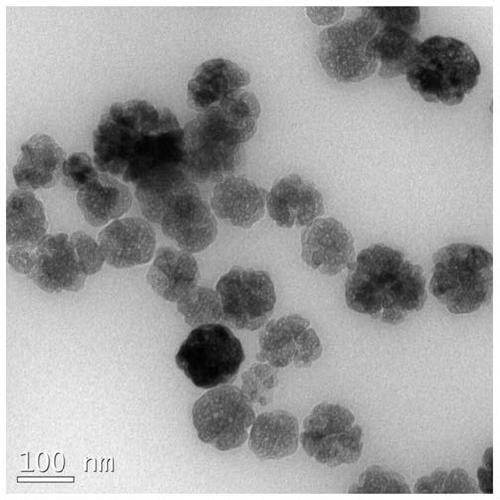
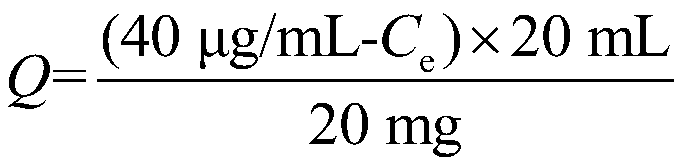Molecularly imprinted magnetic nanospheres of dopamine and its metabolites and its preparation method and application
A technology of magnetic nanospheres and molecular imprinting, applied in chemical instruments and methods, other chemical processes, alkali metal compounds, etc., to achieve the effect of simple preparation method, uniform particle size and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of dopamine and its metabolite molecularly imprinted magnetic nanospheres, comprising the following steps:
[0036] 1) Place 0.30g of ferric chloride, 1.00g of sodium acetate, 40mg of sodium polyacrylate, 10mL of diethylene glycol and 5mL of ethylene glycol in a reaction kettle, and react at 160°C for 5h. After the reaction is completed, use ultrapure water to wash the reaction product. to neutrality and vacuum drying to obtain carboxyl functionalized magnetic nanospheres;
[0037] The vacuum drying temperature is 40°C, the pressure is 0.04MPa, and the drying time is 4h.
[0038] 2) Add 150 mg of the carboxyl-functionalized magnetic nanospheres and two epinephrine metabolites obtained in step 1) into 30 mL of phosphate buffer to obtain a dual-template-carrier complex;
[0039] The two epinephrine metabolites are DA and DOPAC, the dosage of DA is 20 mg, and the dosage of DOPAC is 30 mg; the phosphate buffer is a mixed solution of sodium dihydrogen pho...
Embodiment 2
[0056] A preparation method of dopamine and its metabolite molecularly imprinted magnetic nanospheres, comprising the following steps:
[0057] 1) Place 0.40g of ferric chloride, 1.50g of sodium acetate, 50mg of sodium polyacrylate, 12mL of diethylene glycol and 6mL of ethylene glycol in a reaction kettle, and react at 180°C for 6 hours. The product was washed until neutral, and vacuum-dried to obtain carboxyl-functionalized magnetic nanospheres; the vacuum drying temperature was 47° C., the pressure was 0.06 MPa, and the drying time was 5 h.
[0058] 2) adding 300 mg of the carboxyl-functionalized magnetic nanospheres and two epinephrine metabolites obtained in step 1) into 50 mL of phosphate buffer to obtain a dual template-carrier complex;
[0059] The two epinephrine metabolites are DA and DOPAC, and the dosages of the two are 30 mg and 20 mg respectively; the phosphate buffer is an aqueous solution of sodium dihydrogen phosphate and disodium hydrogen phosphate, with a con...
Embodiment 3
[0075] A preparation method of dopamine and its metabolite molecularly imprinted magnetic nanospheres, comprising the following steps:
[0076] 1) Put 0.50g of ferric chloride, 2.50g of sodium acetate, 80mg of sodium polyacrylate, 20mL of diethylene glycol and 10mL of ethylene glycol in a reaction kettle, and react at 200°C for 10h. After the reaction, use ultrapure water to react. The product was washed until neutral, and vacuum-dried to obtain carboxyl-functionalized magnetic nanospheres; the vacuum drying temperature was 60° C., the pressure was 0.08 MPa, and the drying time was 7 hours.
[0077] 2) Add 200 mg of the carboxyl-functionalized magnetic nanospheres and two epinephrine metabolites obtained in step 1) into 40 mL of phosphate buffer to obtain a dual template-carrier complex;
[0078] The two epinephrine metabolites are DA and DOPAC, and the dosage of both is 30 mg; the phosphate buffer is a mixed solution of sodium dihydrogen phosphate and disodium hydrogen phosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com