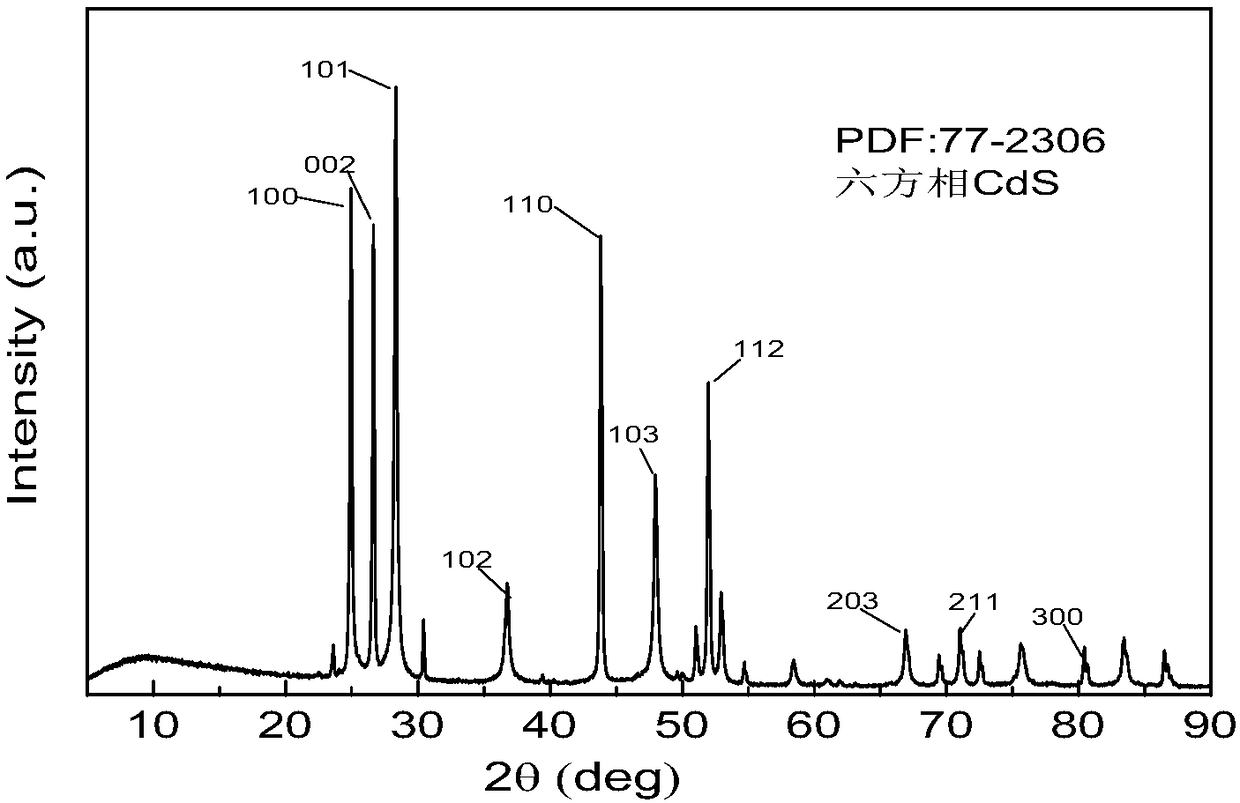
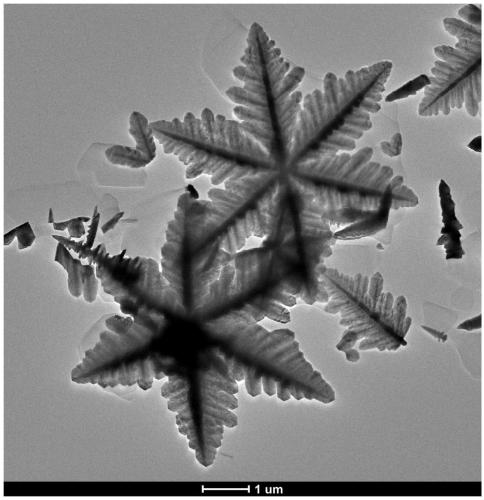
CdS nano-snowflake photocatalyst as well as preparation method and application thereof
A photocatalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of difficult recovery and separation, less exposure of high catalytic activity surfaces, and low utilization of sunlight.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Take by weighing cadmium acetate dihydrate and ammonium thiocyanate respectively in a ratio of 2:3 (cadmium acetate dihydrate: ammonium thiocyanate) by mass ratio to form a mixture, then take 1 part of acetic acid dihydrate in proportion by weight Cadmium + ammonium thiocyanate, dissolved in 100 parts of deionized water, stirred for 30min±5min to dissolve completely, then continued to add 0.5 parts of glacial acetic acid, continued to stir evenly for 10±5min, then poured into the hydrothermal reaction kettle, the filling degree was controlled 80% of the volume of the reaction kettle, then seal the hydrothermal reaction kettle, put it into a dry box, control the hydrothermal temperature at 200°C, and control the reaction time at 20h.
[0027] (2) Naturally cool to room temperature after the reaction finishes, the resulting product is repeatedly washed to neutrality with absolute ethanol, then suction filtered and dried to obtain CdS nano snowflake photocatalyst powder...
Embodiment 2
[0032] (1) Take cadmium acetate dihydrate and ammonium thiocyanate respectively according to the mass ratio range of 1:1 (cadmium acetate dihydrate: ammonium thiocyanate) to form a mixture, and then take 1 part of acetic acid dihydrate in proportion by weight Cadmium + ammonium thiocyanate, dissolved in 100 parts of deionized water, stirred for 30min±5min to dissolve completely, then continued to add 0.5 parts of glacial acetic acid, continued to stir evenly for 10±5min, then poured into the hydrothermal reaction kettle, the filling degree was controlled 50% of the volume of the reaction kettle, then seal the hydrothermal reaction kettle, put it into a dry box, control the hydrothermal temperature at 150°C, and control the reaction time at 10h.
[0033] (2) Naturally cool to room temperature after the reaction finishes, the resulting product is repeatedly washed to neutrality with absolute ethanol, then suction filtered and dried to obtain CdS nano snowflake photocatalyst powde...
Embodiment 3
[0035](1) Take cadmium acetate dihydrate and ammonium thiocyanate respectively in a ratio of 2:5 (cadmium acetate dihydrate:ammonium thiocyanate) by mass ratio to form a mixture, and then take 1.2 parts of dihydrate by weight Cadmium acetate + ammonium thiocyanate, dissolved in 100 parts of deionized water, stirred for 30min±5min to dissolve completely, then continued to add 0.7 parts of glacial acetic acid, continued to stir evenly for 10±5min, then poured into the hydrothermal reaction kettle, the filling degree Control the volume of the reaction kettle at 60%, then seal the hydrothermal reaction kettle, put it into a drying oven, control the hydrothermal temperature at 220°C, and control the reaction time at 15h.
[0036] (2) Naturally cool to room temperature after the reaction finishes, the resulting product is repeatedly washed to neutrality with absolute ethanol, then suction filtered and dried to obtain CdS nano snowflake photocatalyst powder;
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com