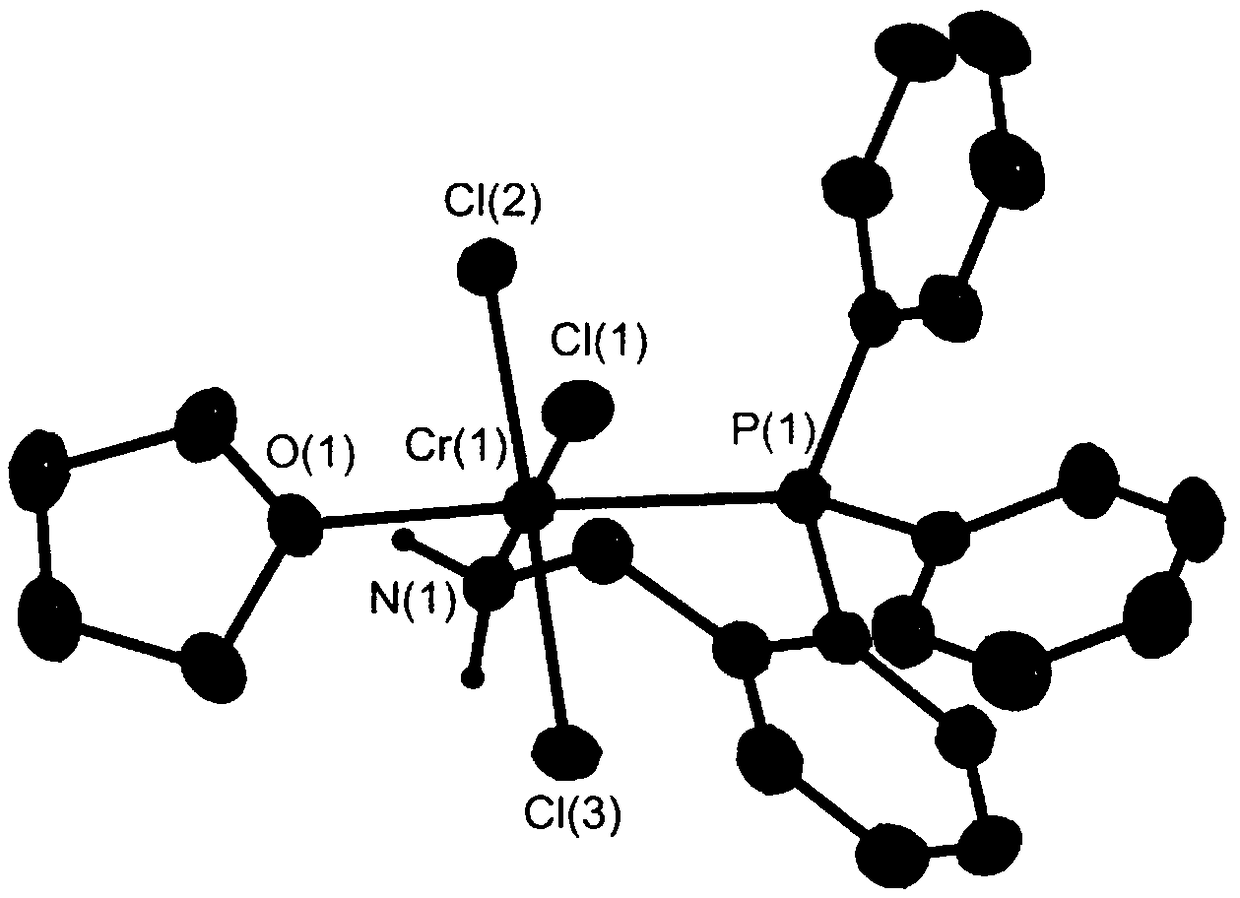
Phosphine nitrogen coordination type metal catalyst and application thereof
A metal catalyst, coordination technology, applied in the direction of catalyst, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc. catalyst system and other problems, to achieve the effect of high catalytic activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 A kind of method of producing 1-hexene and 1-octene by ethylene oligomerization
[0036] Freshly prepare 20 mL of toluene solution of the phosphine nitrogen coordination type metal catalyst shown in the formula II in the glove box, the molar concentration of the metal catalyst is 0.25 mmol / L, and set aside.
[0037] After installation and commissioning of the autoclave, preheat to 100°C, vacuum dry for 5 hours, cool to room temperature, replace with ethylene atmosphere, inject 60mL of toluene solution, 1mL of methylaluminoxane toluene solution (1.5mol / L), formula II 20mL of the toluene solution of the phosphine-nitrogen coordination metal catalyst shown was rapidly heated to 80°C, while raising the ethylene pressure to 4MPa, stirring at 500rpm, and maintaining for 60min.
[0038] Rapidly cool the reaction system to 0°C, release the pressure, add a mixture of 150mL absolute ethanol and 5mL 10% hydrochloric acid to quench, oscillate, let stand, add 2g n-hepta...
Embodiment 2
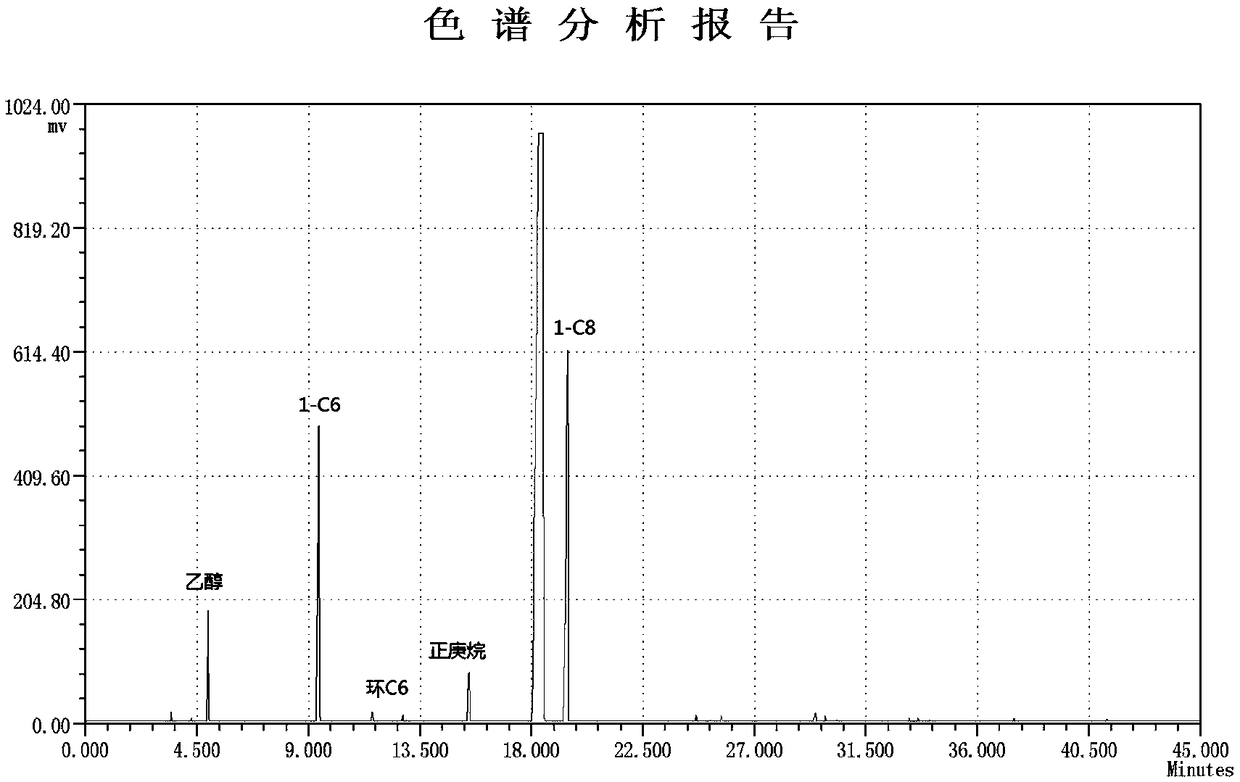
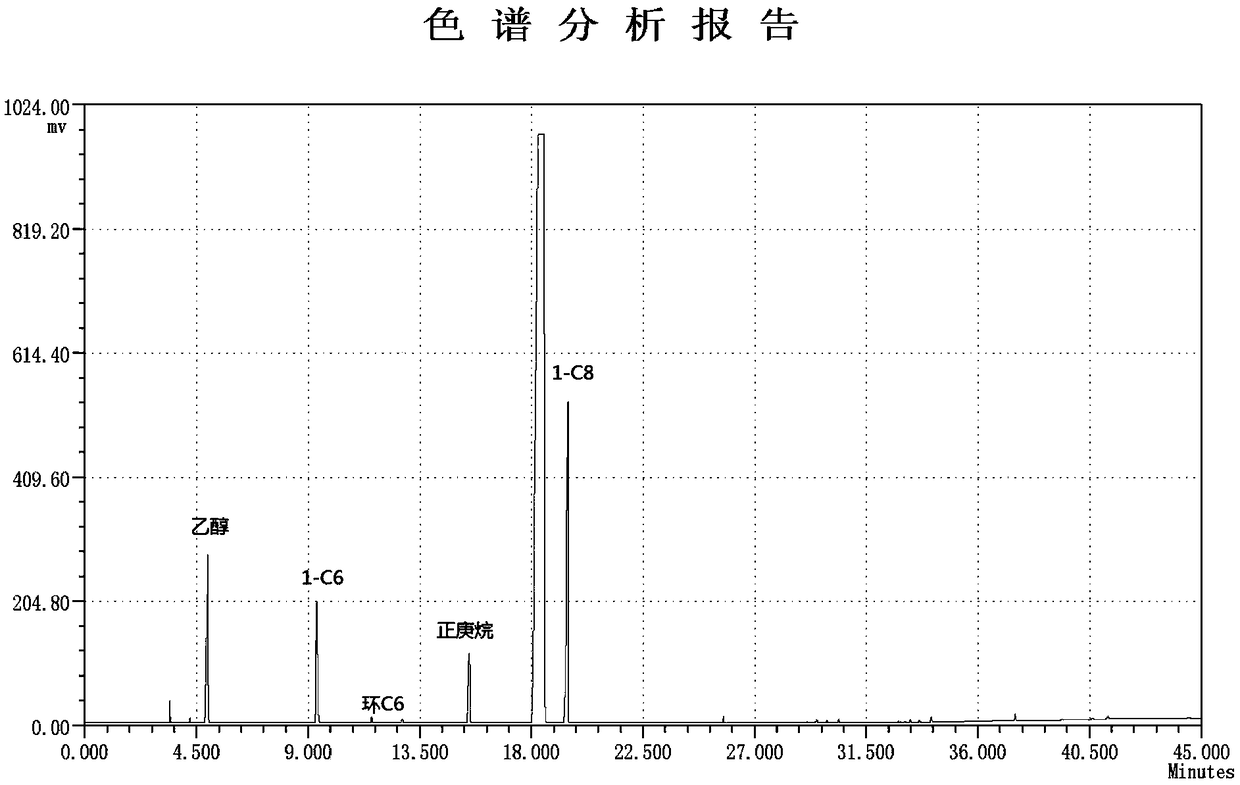
[0043] The reaction temperature in Example 1 was adjusted from 80°C to 70°C, and other operating conditions were unchanged, and the catalyst activity calculated by the internal standard method was 12.6×10 6 g / (mol Cr h), the product composition is recorded in Table 1, and the gas chromatographic analysis of the product is shown in the attached figure 1 .
Embodiment 3
[0045] The reaction temperature in Example 1 was adjusted from 80°C to 60°C, and other operating conditions were unchanged, and the catalyst activity calculated by the internal standard method was 16.5×10 6 g / (mol Cr h), the product composition is recorded in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com