Hypervolume stable lithium battery and preparation method thereof
A lithium battery and super-capacity technology, applied in secondary batteries, battery pack components, circuits, etc., can solve problems such as lack of lithium ion conductivity, heat shrinkable diaphragm, thermal safety issues, etc., to achieve a wider operating temperature range, Good electrical conductivity and the effect of improving the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
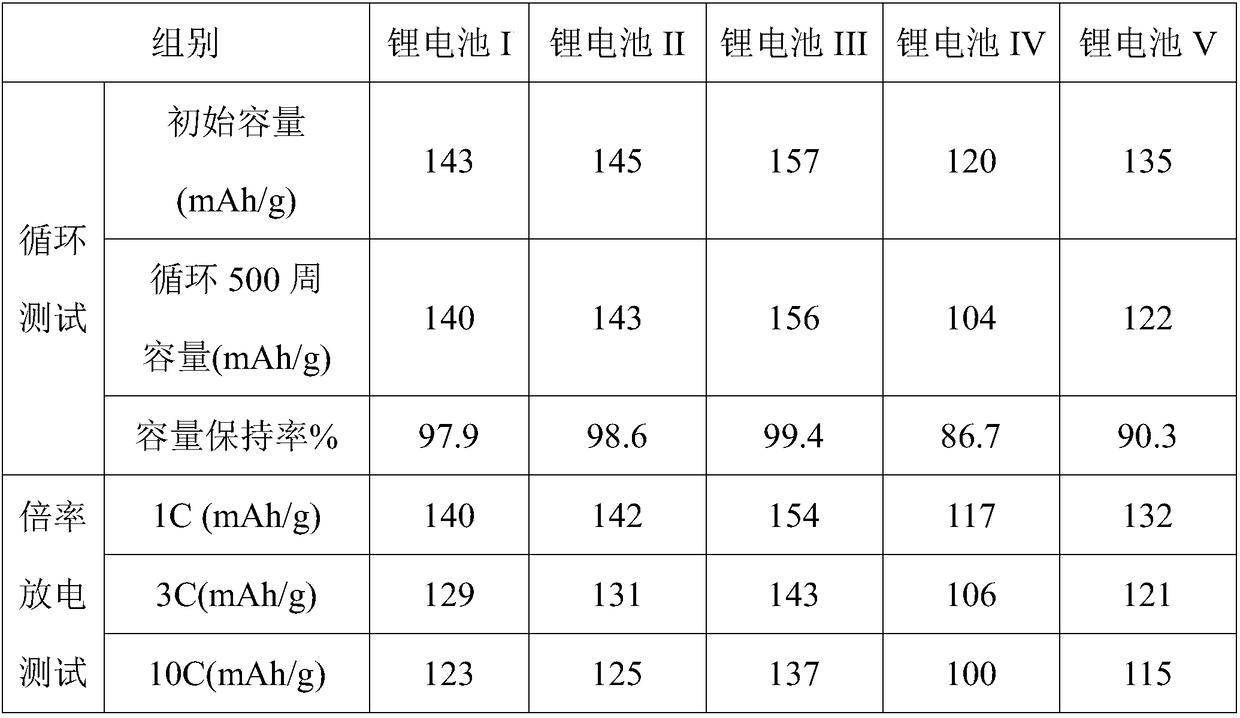
Examples
Embodiment 1
[0025] The preparation of embodiment 1 ultra-capacity stable lithium battery I
[0026] Step 1. Preparation of non-aqueous electrolyte: LiSO 3 CF 3 11 parts, 55 parts of room temperature ionic liquid electrolyte (of which [(CH 3 ) 3 NC 6 h 13 ]N(CF 3 SO 2 ) 2 50 parts of ionic liquid, 1 part of ethylene carbonate, 1.75 parts of ethyl methyl carbonate, 1.25 parts of 4-trifluoromethyl ethylene carbonate, 1 part of ethyl difluoroacetate), 6 parts of composite additives (including vinyl sulfite 1.92 parts of ester, (C 6 h 3 F)O 2 B(C 6 h 3 f 2 ) 1.8 parts, 0.9 parts of triisopropylphenyl phosphate, 1.2 parts of cyclohexylbenzene, and 0.48 parts of nano-lithium carbonate), add them into a Erlenmeyer flask with a ground mouth, mix well, and stir until LiSO 3 CF 3 Completely dissolve to obtain a non-aqueous electrolyte, and the nano-lithium carbonate is prepared by the following method: adding ethyl acetoacetate to a lithium hydroxide aqueous solution with a molar conc...
Embodiment 2
[0031] Embodiment 2 Preparation of super-capacity stable lithium battery II
[0032] Step 1. Preparation of non-aqueous electrolyte: LiAsF 6 19 parts, 75 parts of room temperature ionic liquid electrolyte (of which [(CH 3 ) 3 NC 6 h 13 ] CF 3 SO 2 50 parts of ionic liquid, 7.5 parts of ethylene carbonate, 11.25 parts of ethyl methyl carbonate, 3.75 parts of 4-trifluoromethyl ethylene carbonate, 2.5 parts of ethyl difluoroacetate), 21 parts of composite additives (including vinyl sulfite 6.3 parts of ester, (C 6 f 4 )O 2 B(C 6 f 5 ) 4.62 parts, 3.36 parts of triisopropylphenyl phosphate, 4.2 parts of cyclohexylbenzene, 2.25 parts of nano-lithium carbonate), add to the Erlenmeyer flask with grinding mouth, mix well, and stir until LiAsF 6 Completely dissolve to obtain non-aqueous electrolyte;
[0033] The nano-lithium carbonate is prepared by the following method: ethyl acetoacetate is added to an aqueous solution of lithium hydroxide having a molar concentration of ...
Embodiment 4
[0045] Embodiment 4 comparative example
[0046] Equipment and operation are the same as embodiment 3, and the difference is that LiPF 6 The organic solvent solution replaces the non-aqueous electrolyte solution to obtain a super-capacity stable lithium battery IV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com