Compound with antibacterial effect and application of compound in preparing antibacterial agent
A compound and drug technology, applied in the preparation of antibacterial drugs, in the field of compounds with antibacterial effects, can solve the problems of poor water solubility, unstable chemical sites, and low bioavailability of andrographolide, and achieve the improvement of antibiotic antibacterial / Bacteriostatic effect, significant antibacterial/bacteriostatic activity, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
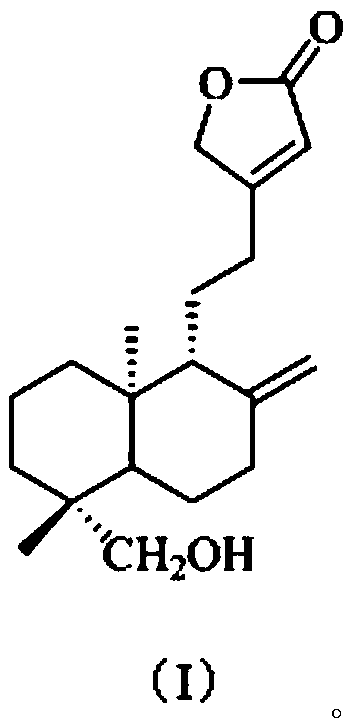
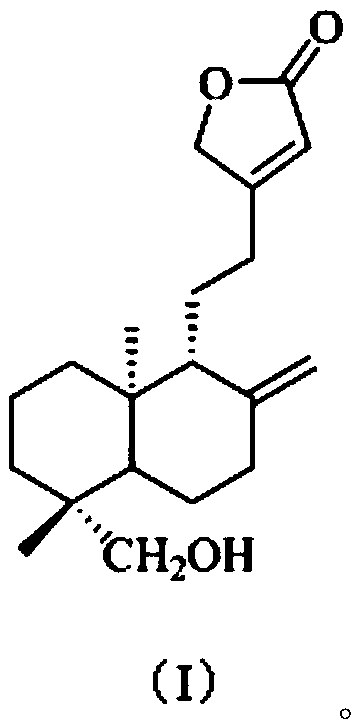
[0038] The present embodiment provides a kind of preparation method of the compound shown in formula (I), comprises the following steps:
[0039] S1. According to the method under the Fuke Qianjin Capsule in the Pharmacopoeia of the People's Republic of China [1]Extract from Zhuzhou Qianjin Pharmaceutical Co., Ltd. and prepare 1.5kg of Fuke Qianjin Prescription Extract Dry Cream (FKQJ). This dry paste was extracted 8 times with 3 times the volume of EtOAc to obtain an EtOAc extract (FKQJE, 156g);
[0040] S2. Take the EtOAc extract (FKQJE, 142.2g) and mix the sample with silica gel, then go through silica gel (1kg, 200-300 mesh) column chromatography, cyclohexane-EtOAc (9:1, 8:2, 7:3, 6:4 , 5:5, v / v) gradient elution, TLC detection and merging of similar fractions, a total of 10 fractions were obtained, respectively named: Fr.1, Fr.2, Fr.3, Fr.4, Fr. 5. Fr.6, Fr.7, Fr.8, Fr.9, Fr.10, spare;
[0041] S3. the fraction Fr.5 (11g) collected in step S2 is removed pigment (MeOH:H...
Embodiment 2
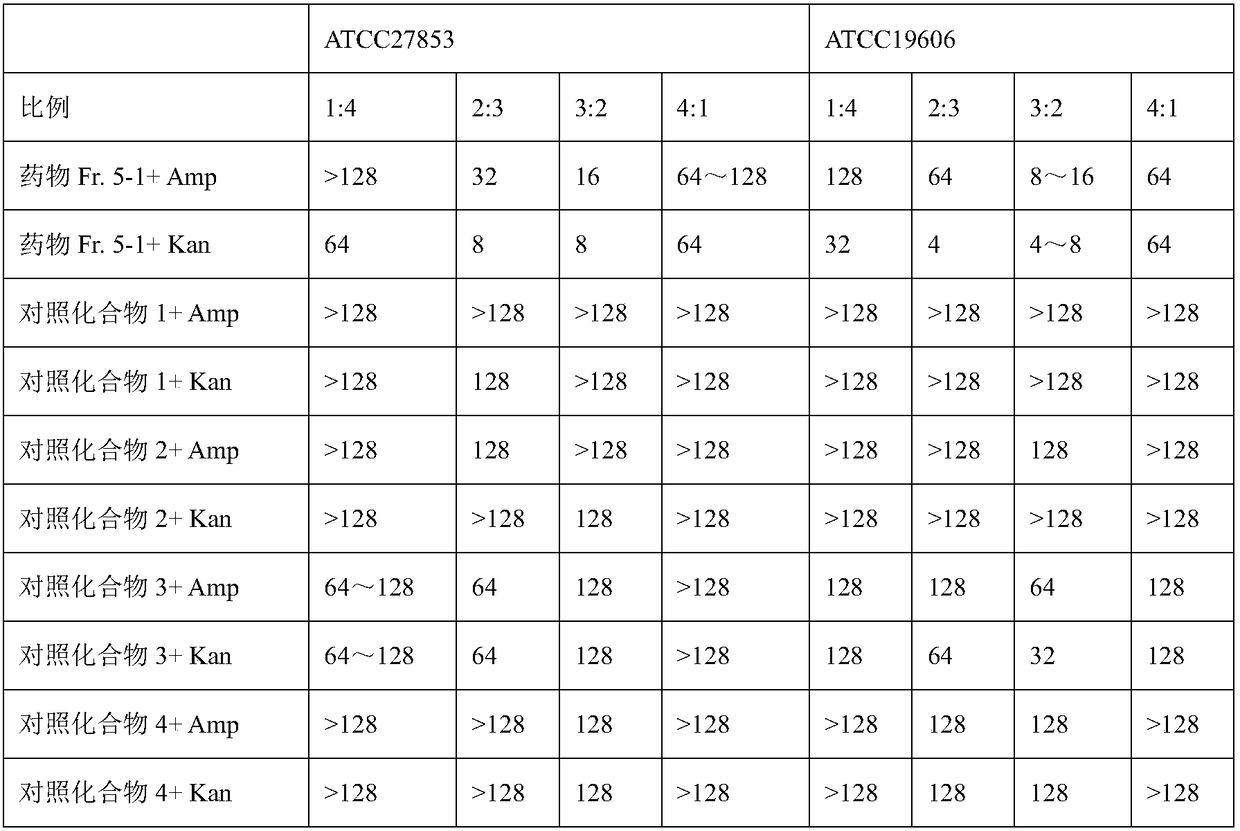
[0051] Use Escherichia coli (ATCC25922), Staphylococcus aureus (ATCC29213), Enterococcus faecalis (ATCC29212), Pseudomonas aeruginosa (ATCC27853) and Acinetobacter baumannii (ATCC19606) as test bacteria, refer to CLSI microplate The antibacterial / bacteriostatic activity of the compounds were detected and verified by the method.
[0052] 1. Experimental method
[0053] 1) Bacterial culture: Cultivate the experimental bacteria with Mueller-Hinton (MH) broth medium, and when it grows for 8-12 hours to about 0.5 Mcfarland concentration (1×10 8 CFU) for backup. A sample solution and a positive control solution with a certain concentration were prepared, and the positive control was ampicillin (Amp) and kanamycin (Kan) two (water soluble).
[0054] 2) Prepare samples and dilute bacteria solution. The samples (drug Fr.5-1 and control compound 1, 2, 3 or 4) were prepared at 2000 μg / mL, and all were dissolved in DMSO. Dilute the bacterial solution reasonably to ensure that the fina...
Embodiment 3
[0070] Escherichia coli (ATCC25922), Staphylococcus aureus (ATCC29213) and Enterococcus faecalis (ATCC29212) were used as test bacteria to test the stability of the drug Fr.5-1 with reference to the microplate method of CLSI.
[0071] 1. Experimental method
[0072] The drug Fr.5-1 together with the reference compounds 1 to 4 in Example 2, ampicillin (Amp) and kanamycin (Kan) are all made into a 1g / mL mother solution with dmso, stored at room temperature for half a year, and detected Its MIC values for Escherichia coli (ATCC25922), Staphylococcus aureus (ATCC29213) and Enterococcus faecalis (ATCC29212).
[0073] Concrete detection method is as embodiment 2.
[0074] 2. Experimental results
[0075] The experimental results are shown in Table 2.
[0076] Table 2 MIC values (μg / mL) of 5 strains of test bacteria:
[0077]
[0078] The results show that the drug Fr.5-1 has better stability than the control compounds 1 to 4, long-term storage at room temperature h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com