Dasatinib grafted polymer micelle, its freeze-dried powder injection, preparation method and application
A technology of freeze-dried powder injection and polymer glue, which is applied in the field of dasatinib grafted polymer micelles, which can solve the problems of slow release and absorption of dasatinib, limited improvement of bioavailability, and long preparation process, etc. problems, to achieve the effects of strong operability and repeatability, improved bioavailability, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of hyaluronic acid-ursodeoxycholic acid amphiphilic graft polymer
[0031] Dissolve 1 g of ursodeoxycholic acid in N-N, dimethylformamide, add 500 mg of N, N'-dicyclohexylcarbodiimide and 250 mg of N-hydroxysuccinimide to activate for 4 hours, then add 5 ml of ethylenediamine, React for 12 hours under the protection of nitrogen. After the reaction, remove the insoluble by-products by filtration, add water to the supernatant to precipitate the product, and collect the precipitate to obtain ursodeoxycholic acid with a free amino group at one end. Dissolve 1g of hyaluronic acid in formamide, add 403mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 200mg of N-hydroxysuccinimide for activation for 30 minutes, then add 100mg Ursodeoxycholic acid with a free amino group at one end was stirred and reacted at room temperature for 20 hours. After the reaction was completed, the reaction solution was transferred to a dialysis bag for dialysis fo...
Embodiment 2
[0037] (1) Preparation of Dasatinib grafted polymer micelles
[0038] Weigh 25 mg of the hyaluronic acid-ursodeoxycholic acid amphiphilic graft polymer prepared in step (1) of Example 1, add 5 ml of deionized water, and stir to obtain a 5 mg / ml graft polymer micelle solution; Take 9 mg of dasatinib and dissolve it in N,N-dimethylformamide to obtain a dasatinib solution. Under high-speed stirring, add the dasatinib solution to the above-mentioned grafted polymer micelle solution, after ultrasonication with a 200W probe for 20 minutes, dialyze with deionized water overnight, and pass through a 0.45 μm filter membrane to obtain the grafted dasatinib type polymer micellar solution. In view of the presence of free carboxyl groups on the surface of the micelles, CaCl was slowly added dropwise during the stirring process. 2 solution, Na 2 HPO 4 solution, a layer of insoluble Ca is formed on the surface of the micelles by charge interaction 3 (PO 4 ) 2 The mineralized layer was...
Embodiment 3
[0042] (1) Preparation of Dasatinib grafted polymer micelles
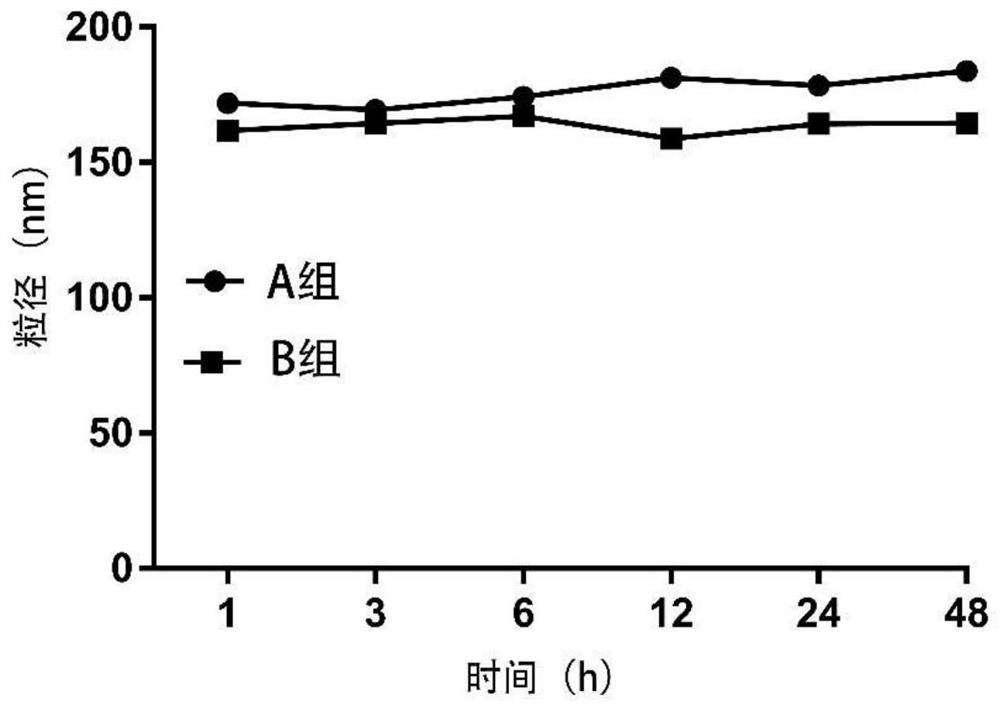
[0043] Weigh 25 mg of the hyaluronic acid-ursodeoxycholic acid amphiphilic graft polymer prepared in step (1) of Example 1, add 5 ml of deionized water, stir to obtain 5 mg / ml to obtain the graft polymer micelle solution ; 9 mg of dasatinib was dissolved in N, N-dimethylformamide to obtain a dasatinib solution. Under high-speed stirring, the dasatinib solution was added to the above-mentioned grafted polymer micelle solution, and after homogenization by a high-pressure homogenizer (homogeneous pressure: 750Pa; number of homogenization cycles: 5 times), deionized Dialyze with water overnight and pass through a 0.45 μm filter membrane to obtain a dasatinib grafted polymer micelle solution. The micelles have an average particle size of 166.4±2.5nm and a Zeta potential of -20.31±1.12mV. The drug loading of dasatinib was 14.9%.
[0044] (2) Preparation of Dasatinib Grafted Polymer Micelle Freeze-dried Powder Injectio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com