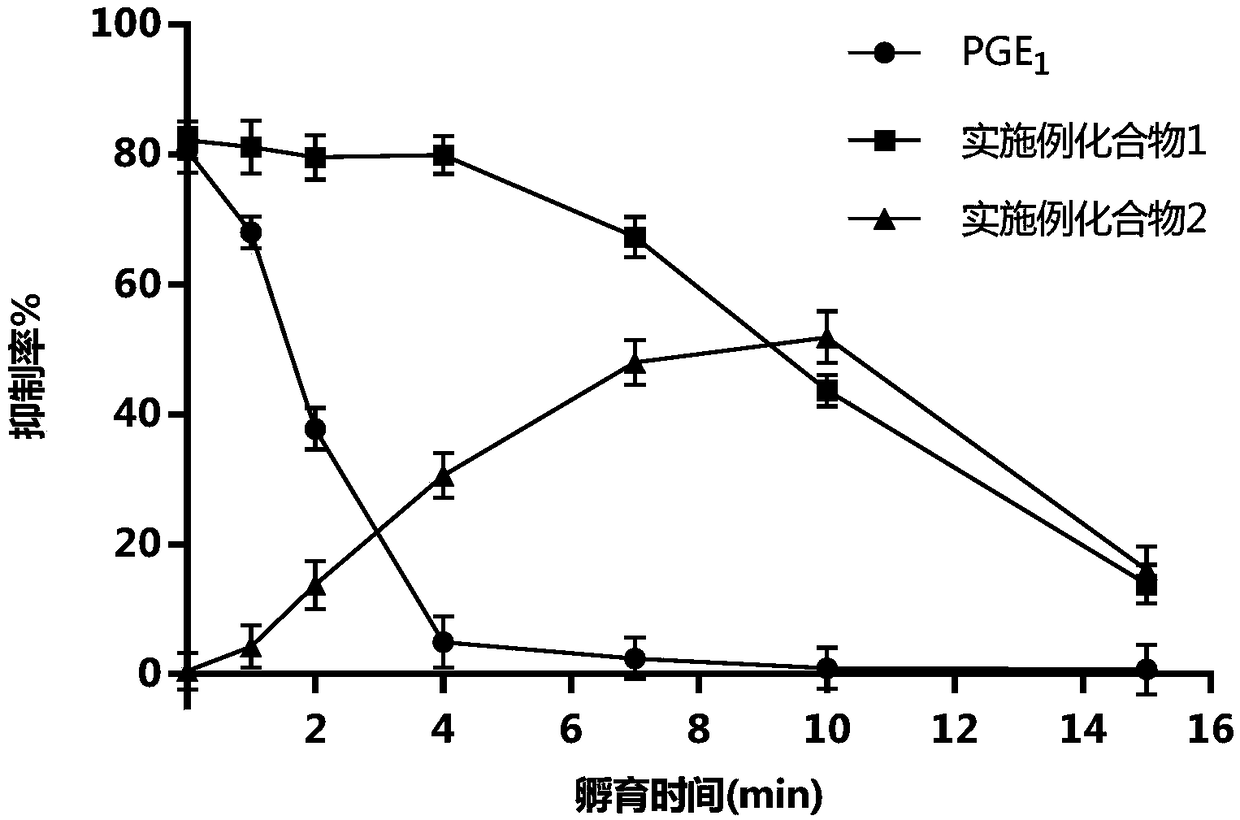
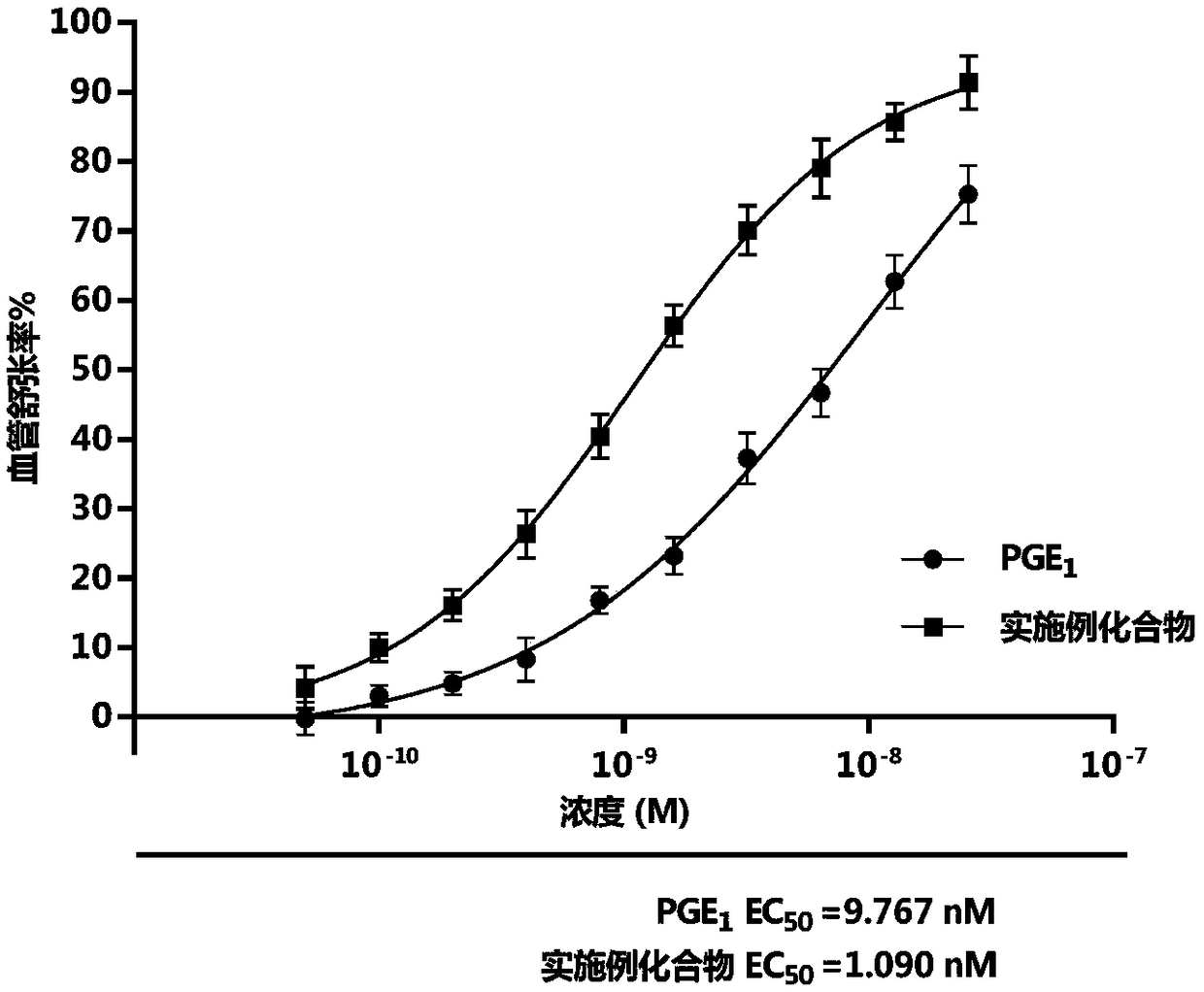
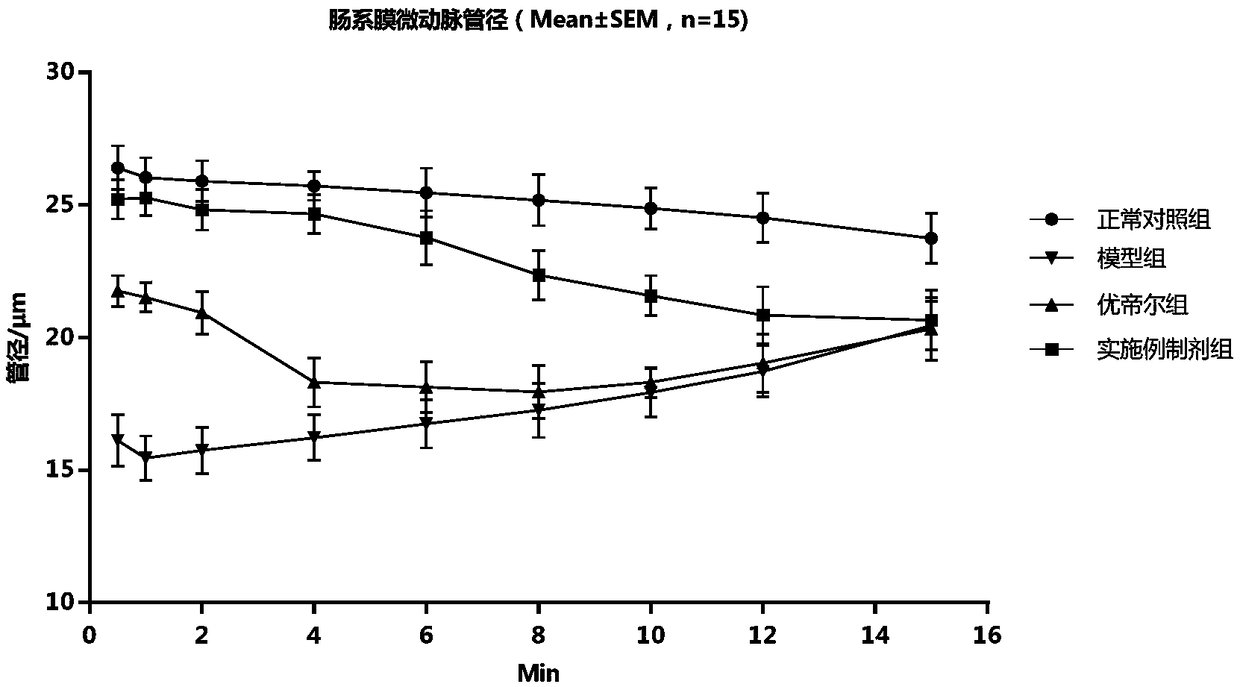
Prostaglandin E1 methyl ester injection freeze-dry preparation, and preparation and application of prostaglandin E1 methyl ester injection freeze-dry preparation
A technology of prostaglandins and freeze-dried preparations, which is applied in the field of medicine, can solve problems such as not meeting the requirements of emulsion droplet size, harsh product storage conditions, and low safety risks, achieve optimal drug activity and therapeutic effect, and improve pharmacokinetics Learning behavior, avoiding the effect of vascular injection stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Compound 1 of the present invention ([(1R,2R,3R)-3-hydroxyl-2-(S,E)-3-hydroxyl-1-enyl)-5-oxocyclopentyl]heptanoic acid methyl ester) Synthesis (Prostaglandin E1 methyl ester)
[0054]
[0055] The raw material PGE1 (63mg, 0.18mmol) was added to a three-necked flask, and then the prepared 1M dry THF / Et2O solution was added and stirred to dissolve. Under ice bath conditions, the MeI (26mg, 1M) solution was slowly added dropwise to the reaction solution, After the dropwise addition, KOH (10mg, 0.18mmol) and Bu 4 NBr (6 mg, 0.018 mmol). After the reaction was stirred for 1 h, it was heated to room temperature and monitored by TLC until the reaction was completed. Add 20ml of water to quench the reaction, extract with EtOAc (10mL×3), combine the organic phases, wash with anhydrous Na 2 SO 4 Dry and filter. The filtrate was concentrated under reduced pressure and purified by column chromatography (eluent n-hexane / EA=1 / 1) to obtain the product as a white solid (24.8 mg...
Embodiment 2
[0059] Synthesis of compound 2 ([(1R,2R,3R)-3-hydroxyl-2-(S,E)-3-hydroxyl-1-enyl)-5-oxocyclopentyl]heptanoic acid ethyl ester) ( prostaglandin E1 ethyl ester)
[0060]
[0061] The raw material PGE1 (63mg, 0.18mmol) was added to the three-necked flask, and then the prepared 1M dry THF / Et 2 The O solution solution was stirred and dissolved, and under the condition of ice bath, EtBr (20mg, 1M) solution was slowly added dropwise to the reaction solution, and after the dropwise addition, KOH (10mg, 0.18mmol) and Bu 4 NBr (6 mg, 0.018 mmol). After the reaction was stirred for 1 h, it was heated to room temperature and monitored by TLC until the reaction was completed. Add 20ml of water to quench the reaction, extract with EtOAc (10mL×3), combine the organic phases, wash with anhydrous Na 2 SO 4 Dry and filter. The filtrate was concentrated under reduced pressure and purified by column chromatography (eluent: n-hexane / EA=1 / 1) to obtain a white solid product (20.5 mg, 29.8% y...
Embodiment 3
[0066]
[0067] The preparation process is as follows:
[0068] Oil phase: Weigh 2g of soybean oil, add 0.5g of egg yolk phospholipid, 0.5mg of prostaglandin E1 methyl ester, and shear until dissolved at 50°C;
[0069] Water phase: Weigh 90g of water for injection, add 0.75g of glycerin, 12.5g of lactose, 0.01g of sodium oleate, mix well by shearing, adjust the pH to 6.5 with 0.1M sodium citrate, then add 2g of phospholipid, continue to shear for 10min;
[0070] Slowly add the oil phase to the water phase, continue to shear at 50°C for 10 minutes to obtain colostrum, add water to 100mL;
[0071] Put the colostrum through the homogenizer, and homogenize it 8 times under the pressure of 850bar;
[0072] Sterile filter the homogenized emulsion, sub-package, pre-freeze at -40°C for 150 minutes, then vacuumize at -20°C, 80mTorr for 480 minutes, 10°C, 60mTorr for 300 minutes, 40°C, 40mTorr for 360 minutes Minutes, vacuumize and press the cap to obtain the freeze-dried powder in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com