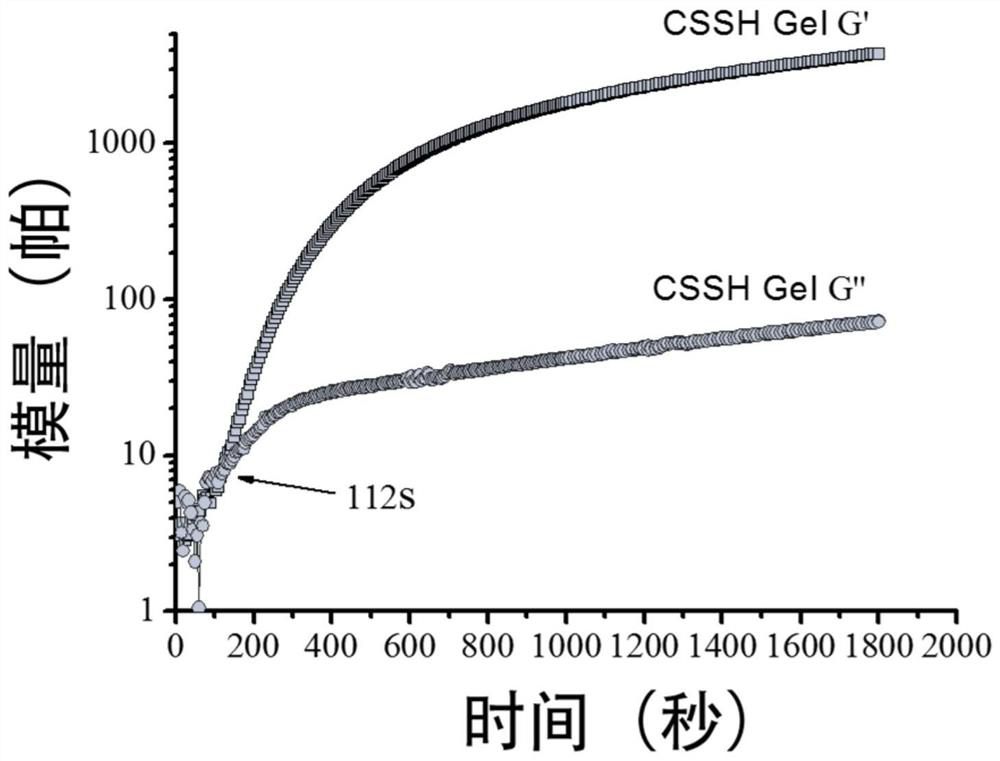
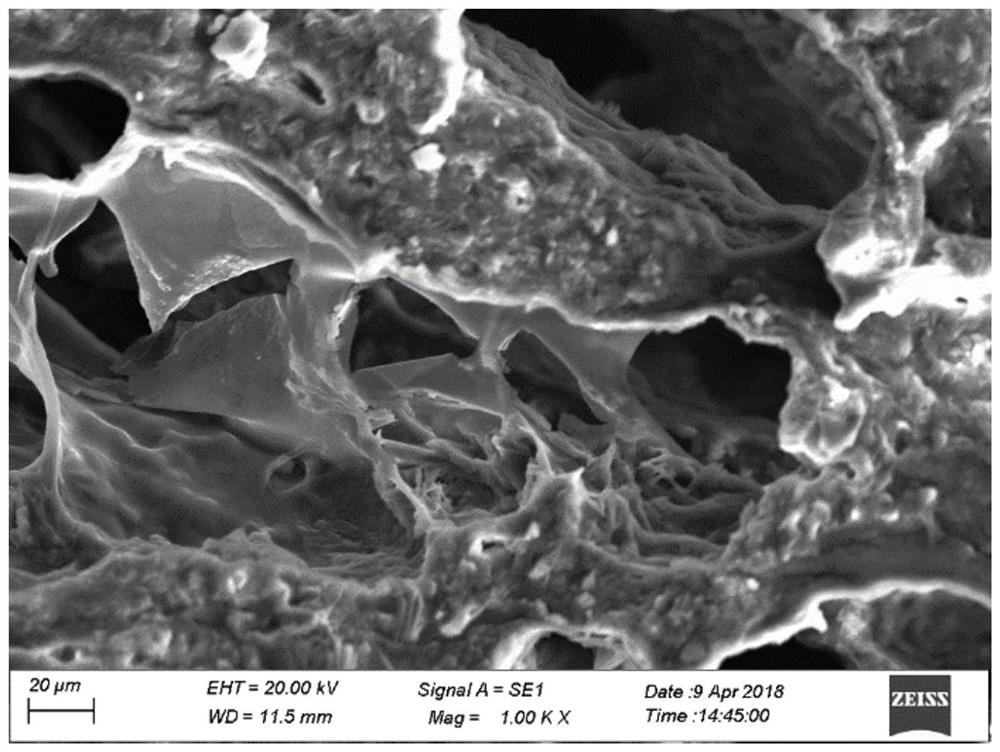
Preparation method and application of a thiolated polysaccharide-based hydrogel capable of in-situ injection molding and its drug carrier
An in situ injection and sulfhydrylation technology, applied in the field of tissue engineering, can solve the problems of poor recovery of injectable hydrogels, uncomfortable surgical operations, poor mechanical strength, etc., to promote skin wound healing, promote epithelial cell growth, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Weigh 1g of chitosan and dissolve it in 100mL of 0.5% (v / v) acetic acid aqueous solution, add 0.9586g of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiethylene Amine hydrochloride (EDAC) and 0.5754g of N-hydroxysuccinimide (NHS), stirred at room temperature for 15 minutes in the dark;
[0054] (2) Add cysteine (the molar ratio of chitosan:cysteine is 3:1) to the reaction system of step (1), add 1mol / L NaOH solution to adjust the system PH=5, room temperature, dark Stir the reaction for 5 hours; sequentially use HCl solution with pH=5.0, HCl solution with pH=5.0 containing 1% (w / v) NaCl and HCl solution with pH=5.0 as the dialysate, each in the dark for 1 day, and dialyze for 3 days in total. day, and finally freeze-dried to obtain the thiolated chitosan (CSSH) sample, which was preserved at 4°C;
[0055] (3) Add the thiolated chitosan prepared in step (2) into deionized water with pH = 8, fully dissolve it under magnetic stirring, put it into a refrigerator at -20°C...
Embodiment 2
[0058] (1) Weigh 1g of chitosan and dissolve it in 100mL of 0.5% (v / v) acetic acid aqueous solution, add 0.9586g of 1-ethyl-3-(3-dimethylaminopropyl)-dicarbonate Imine hydrochloride (EDAC) and 0.5754g of N-hydroxysuccinimide (NHS), stirred at room temperature for 15 minutes in the dark;
[0059] (2) Add cysteine (the molar ratio of chitosan: cysteine is 1:1) to the reaction system of step (1), add 1mol / L NaOH solution to adjust the system PH=5, room temperature, dark Stir the reaction for 5 hours; sequentially use HCl solution with pH=5.0, HCl solution with pH=5.0 containing 1% (w / v) NaCl and HCl solution with pH=5.0 as the dialysate, each in the dark for 1 day, and dialyze for 3 days in total. day, and finally freeze-dried to obtain the thiolated chitosan (CSSH) sample, which was preserved at 4°C;
[0060] (3) Add the thiolated chitosan prepared in step (2) into deionized water with pH = 8, fully dissolve it under magnetic stirring, put it into a refrigerator at -20°C fo...
Embodiment 3
[0063] (1) Weigh 1g of chitosan and dissolve it in 100mL of 0.5% (v / v) acetic acid aqueous solution, add 0.9586g of 1-ethyl-3-(3-dimethylaminopropyl)-dicarbonate Imine hydrochloride (EDAC) and 0.5754g of N-hydroxysuccinimide (NHS), stirred at room temperature for 15 minutes in the dark;
[0064] (2) Add cysteine (the molar ratio of chitosan: cysteine is 1:3) to the reaction system of step (1), add 1mol / L NaOH solution to adjust the pH of the system to 5, room temperature, dark Stir the reaction for 5 hours; sequentially use HCl solution with pH=5.0, HCl solution with pH=5.0 containing 1% (w / v) NaCl and HCl solution with pH=5.0 as the dialysate, each in the dark for 1 day, and dialyze for 3 days in total. day, and finally freeze-dried to obtain a thiolated chitosan (CSSH) sample, which was stored at 4°C;
[0065] (3) Add the thiolated chitosan prepared in step (2) into deionized water with pH = 8, fully dissolve it under magnetic stirring, put it into a refrigerator at -20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com