Preparation method of nitrogen doped carbon nano-array loaded iron phosphide/cobalt phosphide
A nano-array, nitrogen-doped carbon technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of high cost, slow electrochemical hydrogen production reaction kinetics, and low energy conversion efficiency. and other problems, to achieve the effect of low cost and superior hydrogen production performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 CoP 3 / Preparation of FeP@N-C
[0031] 1.1 Preparation of carbon paper
[0032] Prepare 40mL of 1mol / L nitric acid solution in a small beaker, place the small beaker containing the nitric acid solution on a magnetic stirrer for heating in a water bath, cook the carbon paper with acid at 90°C for 1 hour at a medium speed; take out the boiled carbon Paper, mark on carbon paper, take a 50mL small beaker and clean it, and add an appropriate amount of ultrapure water to it, add the marked carbon paper to it, perform ultrasonic cleaning for 10 minutes, and then replace the ultrapure water with ethanol ultrasonic Wash for 5 minutes; then dry in the oven for 12 hours.
[0033] 1.2 Preparation of polyaniline nanoarrays (N-CNAs)
[0034] Take 50mL of ultrapure water into the beaker, place the beaker on a magnetic stirrer and start stirring, take 4.310mL of perchloric acid into the beaker with a pipette gun, then take 0.460mL of aniline solution into the previous bea...
Embodiment 2
[0039] Example 2 CoP 3 / Physicochemical properties of FeP@N-C
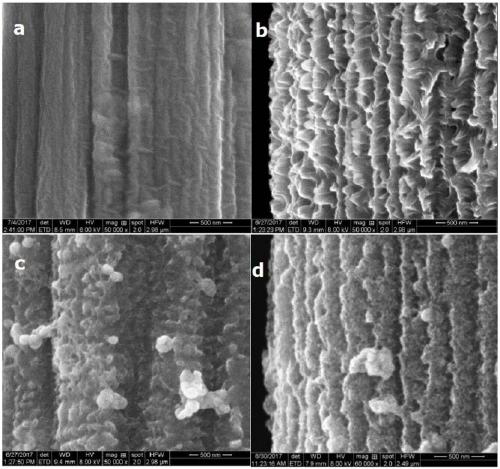
[0040] Intermediate product and final product are observed with scanning electron microscope in each preparation process in embodiment 1, and photo is as follows figure 1 As shown, among them, a is the scanning electron micrograph of carbon paper; b is the scanning electron micrograph of N-CNAs; c is the scanning electron micrograph of PANI-Fe / Co MOF; d is the CoP 3 / FeP@N-C scanning electron microscope image. Comparing Figure a and Figure b, it can be clearly seen that polyaniline grew on the original smooth carbon paper surface during the electrodeposition process, forming a highly consistent nano-array structure. After the cobalt ferrite MOF is grown, it can be seen that the N-CNAs are coated with a layer of cobalt ferrite MOF. At this time, the array is still highly ordered and the morphology is relatively complete. After low-temperature calcination and phosphating, the morphology of the array shrunk compa...
Embodiment 3
[0041] Example 3 CoP 3 Catalytic activity of / FeP@N-C
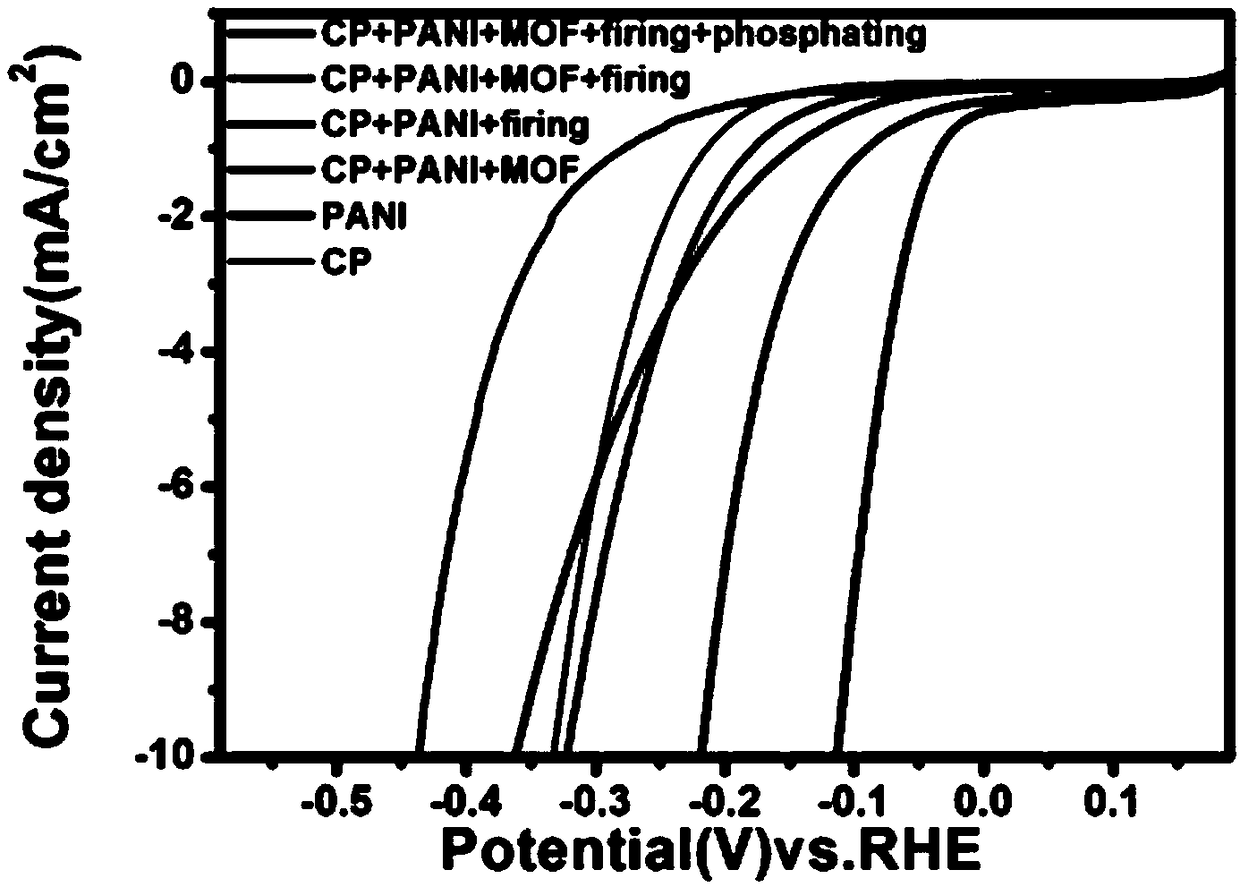
[0042] Both the intermediate product and the final product prepared in Example 1 were placed in 0.5mol / L sulfuric acid, and tested by Shanghai Chenhua Electrochemical Workstation, the potential window was 0~-0.4V, image 3 It is the linear voltammetry scanning curve of each intermediate product and the final product of Example 1, wherein, the materials and processing methods intersecting with the abscissa from left to right are CP+PANI+MOF+firing, PANI, CP, CP+PANI+ MOF, CP+PANI+firing, CP+PANI+MOF+firing+phosphating; CP stands for carbon paper, PANI stands for polyaniline nanoarray, MOF stands for Fe / Co MOF, firing stands for calcination, and phosphating stands for phosphating. By comparison, the CoP 3 The combination of / FeP@N-C has better performance than other materials (carbon paper, N-CNAs, PANI-Fe / Co MOF, etc.), and its initial overpotential is about 50mv and the catalyst surface is built at about 0.11V Current ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com