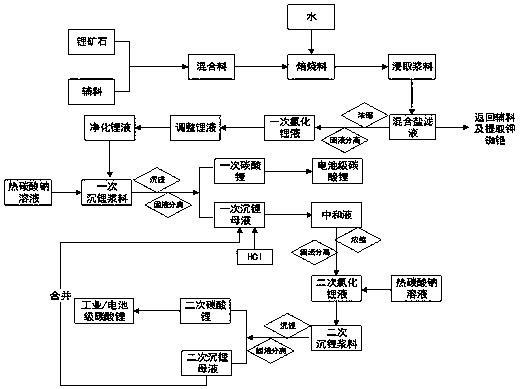
Method for extracting lithium salt from lithium ore
A lithium ore and lithium extraction technology, applied in the direction of lithium carbonate;/acid carbonate, etc., can solve the problems of reduced yield and efficiency, equipment corrosion, large amount of neutralized slag, etc., and achieve high comprehensive utilization rate , the effect of improving yield and easy washing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Weigh 5000g of spodumene with a lithium oxide content of 7.5%, add 4800g of calcium chloride, and mix evenly; roast at 1050°C for 60 minutes to obtain a roasted material; take it out, cool to room temperature, add 12000mL of water, and stir for 30 minutes. Filtration to obtain filtrate and filter residue.
[0047] Table 1 Spodumene data, %
[0048] Li / Li2O
K
Rb
Cs
3.49 / 7.50
0.35
0.10
0.035
[0049] Table 2 filtrate data, g / L
[0050] Li
Na
K
14.52
1.03
1.44
[0051] Table 3 Residue data, %
[0052]
Li
K
Rb
Cs
0.07
0.03
0.0015
0.0007
99.07
91.43
98.50
98.00
[0053] (2) Concentrate the filtrate obtained in step (1), and separate the obtained concentration into solid and liquid. The liquid data are as follows:
[0054] Table 4 Concentrate data, g / L
[0055] LiCl
Na
K
Rb
Cs ...
Embodiment 2
[0065] (1) Weigh 2000g of finely ground β-spodumene with a lithium oxide content of 6.0%, add 2400g of calcium chloride, 1000g of sodium chloride, and potassium chloride mixed salt, and mix evenly; roast at 920°C for 20 minutes to obtain a roasted material; Take it out, after cooling to room temperature, add 4000mL of water, stir for 30min, and filter to obtain filtrate and filter residue.
[0066] Table 6 Spodumene data, %
[0067] Li / Li2O
[0068] Table 7 filtrate data, g / L
[0069] Li
[0070] Table 8 Residue data, %
[0071] Li2CO3
[0072] (2) Concentrate the filtrate obtained in step (1), and separate the obtained concentration into solid and liquid. The liquid data are as follows:
[0073] Table 9 Concentrate data, g / L
[0074] LiCl
[0075] (3) Adjust the concentrated solution obtained in step (2) to a lithium content of 22g / L, add a slight excess of sodium carbonate and sodium hydroxide according to the residual divalent meta...
Embodiment 3
[0081] (1) Weigh 6000g of finely ground spodumene with a lithium oxide content of 5.3%, add 500g of calcium carbonate, 3600g of calcium chloride, and 2000g of sodium chloride, and mix well; roast at 990°C for 60 minutes to obtain a roasted material; take it out and cool After reaching room temperature, 15 L of water was added, stirred for 30 min, and filtered to obtain filtrate and filter residue.
[0082] Table 11 Spodumene data, %
[0083] Li / Li2O
[0084] Table 12 Filtrate data, g / L
[0085] Li
[0086] Table 13 Residue data, %
[0087]
[0088] (2) Concentrate the filtrate obtained in step (1), and separate the obtained concentration into solid and liquid. The liquid data are as follows:
[0089] Table 14 Concentrate data, g / L
[0090] LiCl
[0091] (3) Adjust the concentrated solution obtained in step (2) to a lithium content of 22g / L, add a slight excess of sodium carbonate and sodium hydroxide according to the residual divalent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com