Triazole Schiff base Myricetin derivative containing thioether, preparation method and application thereof
A technology of triazole schiff base and derivatives is applied in the field of preparation of triazole schiff alkaloid myricetin derivatives, can solve problems such as lack of unwinding activity, and achieve the effects of good resistance to plant viruses and excellent inhibition of plant pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
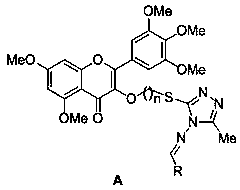
[0032] 3-(3-((5-Methyl-4-((benzylidene)amino)-4H-1,2,4-triazol-3-yl)thio)propoxy)-5,7- Dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-benzopyran-4-one (target compound A 1 ) preparation method, comprising the following steps:
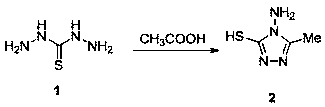
[0033] (1) Preparation of hydrazinothiohydrazide (intermediate 1):
[0034]Add 20mL of 85% hydrazine hydrate and 60mL of water to a three-neck flask equipped with a thermometer, dropping funnel and condenser (the upper port is connected with a tail gas outlet pipe), and control the temperature at about 50°C. Under electromagnetic stirring, 6 mL of CS was added dropwise within 1 h 2 , and then the mixed solution was refluxed at 90° C. for 1 h, cooled to crystallize, suction filtered, and recrystallized in water to obtain colorless needle-like crystals (intermediate 1), yield: 82%.
[0035] (2) Preparation of 4-amino-5-methyl-4H-1,2-4-triazole-3-mercapto (intermediate 2):
[0036] Take 3.18g (0.03mol) of hydrazinothiohydrazide (intermediate 1) in a flask, ad...
Embodiment 2
[0046] 3-(3-((5-methyl-4-((4-methylbenzylidene)amino)-4H-1,2,4-triazol-3-yl)thio)propoxy)- 5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (target compound A 2 ) preparation method, comprising the following steps:
[0047] (1) Preparation of hydrazinothiohydrazide (intermediate 1):
[0048] As in the first (1) step of Example 1.
[0049] (2) Preparation of 4-amino-5-methyl-4H-1,2,4-triazole-3-mercapto (intermediate 2):
[0050] As in embodiment 1 (2) step.
[0051] (3) Preparation of 5-methyl-4-((4-methylbenzylidene)amino)-4H-1,2,4-triazole-3-thiol:
[0052] As in the (3) step of embodiment 1, the difference is that p-tolualdehyde is used as raw material.
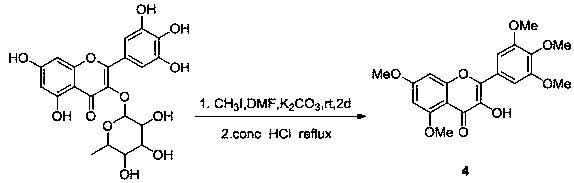
[0053] (4) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin:
[0054] As embodiment 1 (4) step.
[0055] (5) 3-(3-bromopropoxy)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (intermediate 5) Preparation:
[0056] As in step (5) of Example 1.
[0057] (6) 3-(3-((5-methyl-4-((4-methy...
Embodiment 3
[0060] 3-(3-((5-methyl-4-((3-methylbenzylidene)amino)-4H-1,2,4-triazol-3-yl)thio)propoxy)- 5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (target compound A 3 ) preparation method, comprising the following steps:
[0061] (1) Preparation of hydrazinothiohydrazide (intermediate 1):
[0062] As in the first (1) step of Example 1.
[0063] (2) Preparation of 4-amino-5-methyl-4H-1,2,4-triazole-3-mercapto (intermediate 2):
[0064] As in embodiment 1 (2) step.
[0065] (3) Preparation of 5-methyl-4-((3-methylbenzylidene)amino)-4H-1,2,4-triazole-3-thiol
[0066] As in the (3) step of embodiment 1, the difference is that taking m-tolualdehyde as raw material.
[0067] (4) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0068] As embodiment 1 (4) step.
[0069] (5) 3-(3-bromopropoxy)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one (intermediate 5) Preparation:
[0070] As in step (5) of Example 1.
[0071] (6) 3-(3-((5-methyl-4-((3-methylbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com