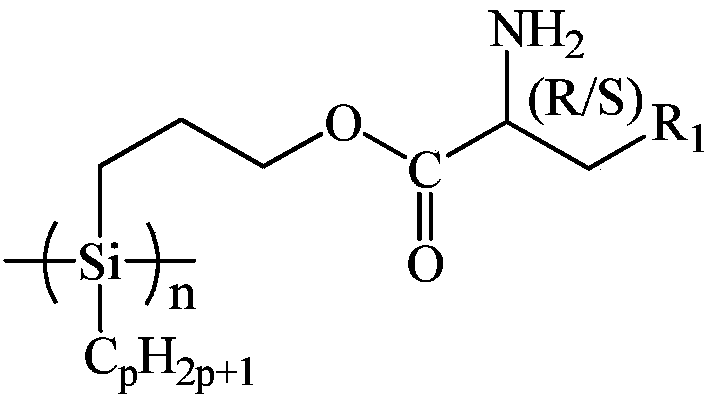
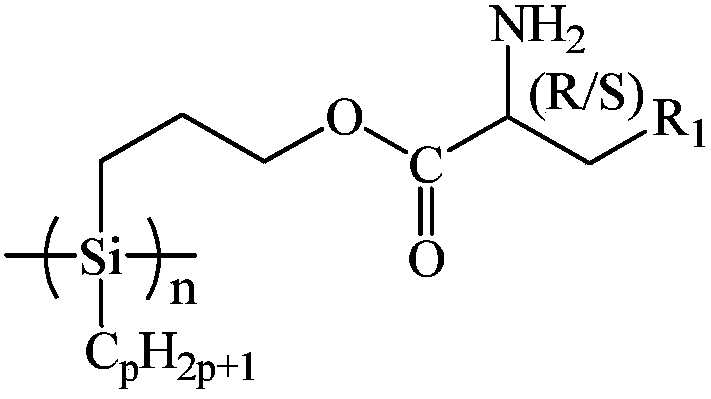
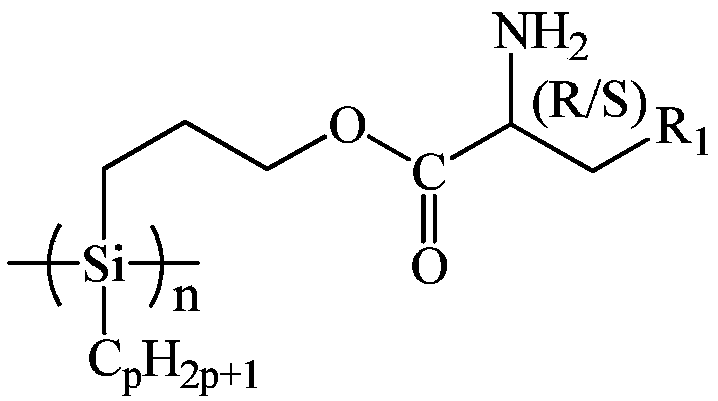
Amino acid spiral polysilane infrared absorption material and preparation method thereof
An amino acid-based, infrared absorption technology, applied in the field of amino acid-based helical polysilane infrared absorption materials and their preparation, can solve problems such as the destruction of side chain functional groups, harsh reaction conditions chlorosilane, etc., to improve unsaturation and thermal stability. , the effect of good solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] At 25°C under Ar atmosphere, add 60.00g of xylene and 3.48g of sodium metal into the reaction kettle, stir and disperse at 135°C for 1h, remove xylene by distillation under reduced pressure, drop to 25°C, add 60.00g of tetrahydrofuran, and add 6.09g of dichlorodecylhydrosilane, reacted at 25°C for 22h, added 360.00g of methanol to remove excess sodium metal, filtered, and washed the filter cake successively with 15.00g of water and methanol each time, washing 3 times to remove residual sodium methoxide, Vacuum drying at 25°C for 1 hour yielded 3.01 g of decylhydrogenpolysilane;
[0041] At 25°C, add 36.50g of L-glutamine and 50.00g of toluene into the reaction kettle, and add 48.50g of p-toluenesulfonic acid, then add 100.00g of allyl alcohol, heat up to reflux, react for 24h, and remove by distillation under reduced pressure. Propyl alcohol, toluene and water produced by reaction, add 100.00g of diethyl ether into the reaction kettle at 25°C, reflux for 0.5h, drop to 2...
Embodiment 2
[0044] At 25°C under Ar atmosphere, add 60.00g of xylene and 3.48g of sodium metal into the reaction kettle, stir and disperse at 135°C for 1h, remove xylene by distillation under reduced pressure, drop to 25°C, add 60.00g of tetrahydrofuran, and add 6.09g of dichlorodecylhydrosilane, reacted at 25°C for 22h, added 360.00g of methanol to remove excess sodium metal, filtered, and washed the filter cake successively with 15.00g of water and methanol each time, washing 3 times to remove residual sodium methoxide, Vacuum drying at 25°C for 1 hour yielded 3.01 g of decylhydrogenpolysilane;
[0045] At 25°C, add 36.50g of D-glutamine and 50.00g of toluene into the reaction kettle, add 48.50g of p-toluenesulfonic acid, then add 100.00g of allyl alcohol, heat up to reflux, react for 24h, and remove by distillation under reduced pressure. Propylene alcohol, toluene and water produced by reaction, add 100.00g diethyl ether into the reaction kettle at 25°C, reflux for 0.5h, drop to 25°C,...
Embodiment 3
[0048] At 25°C under Ar atmosphere, add 60.00g of xylene and 3.48g of sodium metal into the reaction kettle, stir and disperse at 135°C for 1h, remove xylene by distillation under reduced pressure, drop to 25°C, add 60.00g of tetrahydrofuran, and add 6.46g of dichloroundecylhydrosilane, reacted at 25°C for 22h, added 360.00g of methanol to remove excess sodium metal, filtered, washed the filter cake with 15.00g of water and methanol respectively, and washed 3 times to remove residual methanol Sodium, vacuum-dried at 25°C for 1 hour to obtain 3.26 g of undecylhydrogenpolysilane;
[0049] At 25°C, add 36.50g of L-lysine and 50.00g of toluene into the reaction kettle, and add 48.50g of p-toluenesulfonic acid, then add 100.00g of allyl alcohol, heat up to reflux, react for 24h, and remove by distillation under reduced pressure. Propyl alcohol, toluene and water produced by reaction, add 100.00g of diethyl ether into the reaction kettle at 25°C, reflux for 0.5h, drop to 25°C, filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com