Electrocatalyst for sulfur-nitrogen-transition metal co-doped carbon-based oxygen reduction, and preparation method and application thereof
A transition metal and electrocatalyst technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of large particle size and performance gap of electrocatalysts, and achieve the effects of simple steps, convenient operation, and good oxygen reduction catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
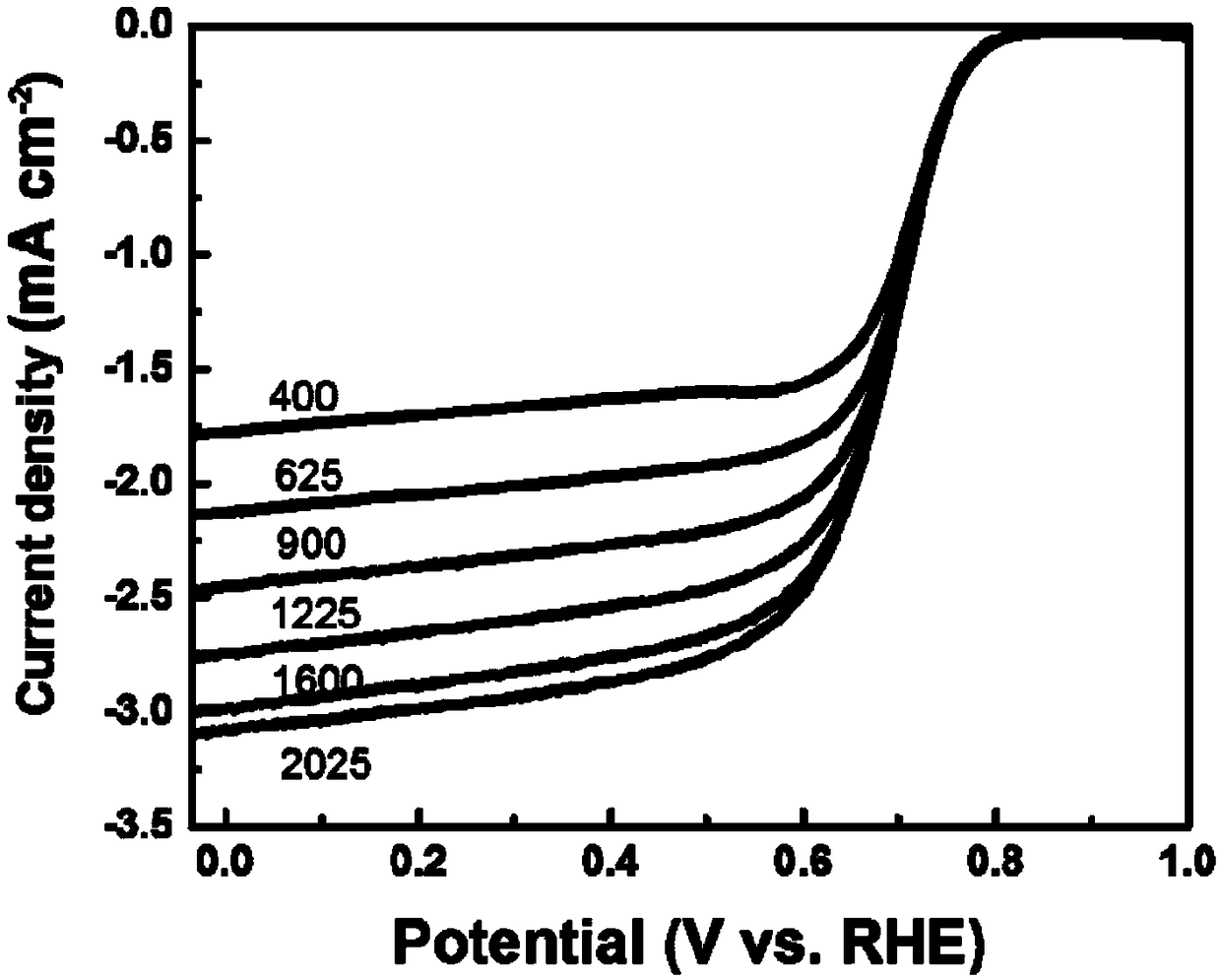
[0039] Put 13.45mmol 2,4-dihydroxybenzaldehyde, 055mmol 2-formylpyridine, 13.45mmol thiosemicarbazide and 14.83mmol cobalt chloride hexahydrate in a container, add 22mL ethanol and stir the reaction for 24h; mix the obtained The solution is placed in a hydrothermal kettle, the reaction temperature is 120°C, and the reaction time is 24h; then the obtained reactant is placed in an oxidation furnace, and the temperature is raised to 280°C at a heating rate of 2°C / min and cooled to room temperature after a constant temperature of 4h; The obtained oxide was placed in a tube furnace, raised to 900°C at a rate of 2°C / min in a nitrogen atmosphere, kept at a constant temperature for 3 hours, and then lowered to room temperature; the product was taken out of the tube furnace, washed with 1M dilute hydrochloric acid, and then washed with Wash with deionized water to neutrality and dry to obtain a carbon-based non-noble metal oxygen reduction electrocatalyst. The resulting product is marke...
Embodiment 2
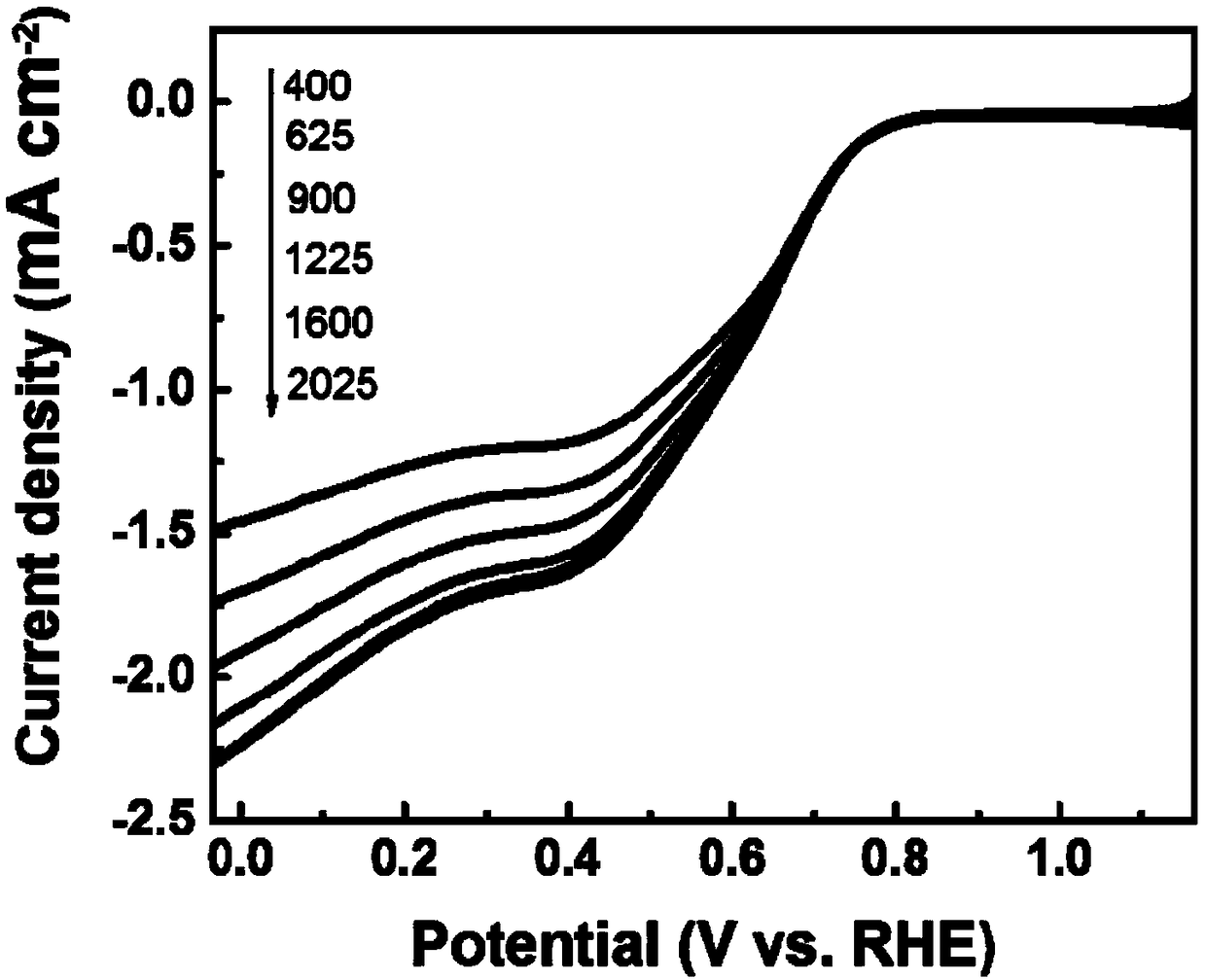
[0043] Put 12.65mmol 3,5-diaminobenzaldehyde, 37.95mmol thiourea, 10.32mmol thiosemicarbazide and 23.65mmol cobalt chloride hexahydrate in a container, add 22mL ethanol and stir the reaction for 12h; In a hydrothermal kettle, the reaction temperature is 150°C, and the reaction time is 20h; then the obtained reactant is placed in an oxidation furnace, the temperature is raised to 300°C at a heating rate of 5°C / min, and the temperature is kept at a constant temperature for 6h and then cooled to room temperature; then the obtained The oxide is placed in a tube furnace, raised to 1000 °C at a rate of 5 °C / min in a nitrogen atmosphere, kept at a constant temperature for 5 hours, and then lowered to room temperature; the product is taken out of the tube furnace, washed with 1M dilute hydrochloric acid, and then deionized Wash with water to neutrality and dry to obtain carbon-based non-noble metal oxygen reduction electrocatalyst. The obtained product is marked as C-2. The appearance ...
Embodiment 3
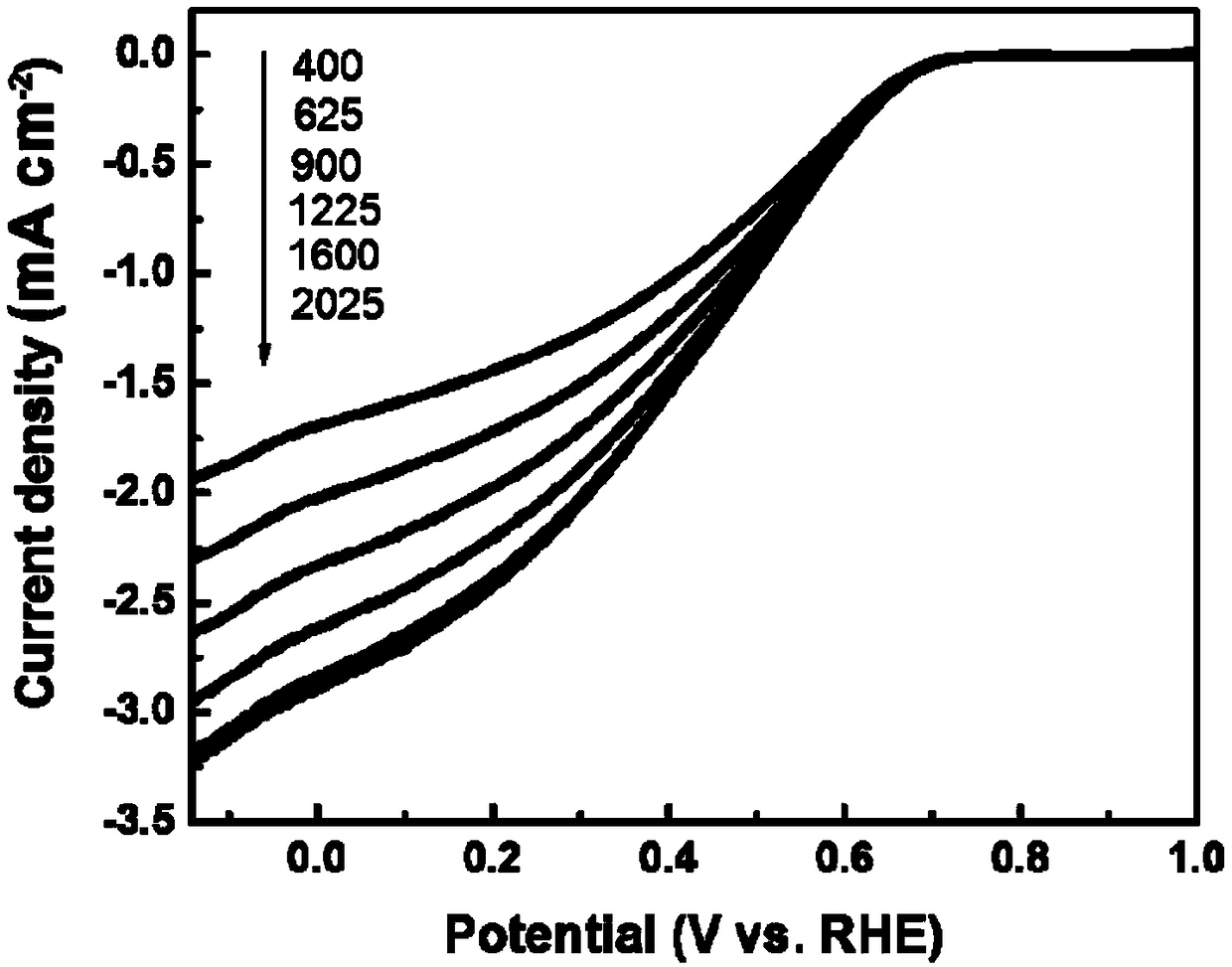
[0047] Put 11.32mmol of p-aminobenzaldehyde, 37.95mmol of thioacetamide and 12.58mmol of ferric chloride in a container, add 12mL of ethanol and 10mL of distilled water and stir the reaction for 18h; place the resulting mixed solution in a hydrothermal kettle, and the reaction temperature 90°C, the reaction time is 24h; then the obtained reactant is placed in an oxidation furnace, the temperature is raised to 260°C at a heating rate of 5°C / min and the temperature is kept at a constant temperature for 5h, and then cooled to room temperature; then the obtained oxide is placed in a tube furnace, In a nitrogen atmosphere, the temperature was raised to 900°C at a rate of 10°C / min and kept at a constant temperature for 4 hours, and then lowered to room temperature; the product was taken out of the tube furnace, washed with 1M dilute hydrochloric acid, then washed with deionized water until neutral, and dried, that is Obtain carbon-based non-noble metal oxygen reduction electrocatalys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com