Spherical Co (II) Co (III) hydrotalcite like material and preparation method thereof
A hydrotalcite and spherical technology, which is applied in the field of spherical CoCo hydrotalcite-like materials and its preparation, can solve problems such as undiscovered, and achieve the effect of dense surface, good sphericity of product, and dense surface of sphere
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
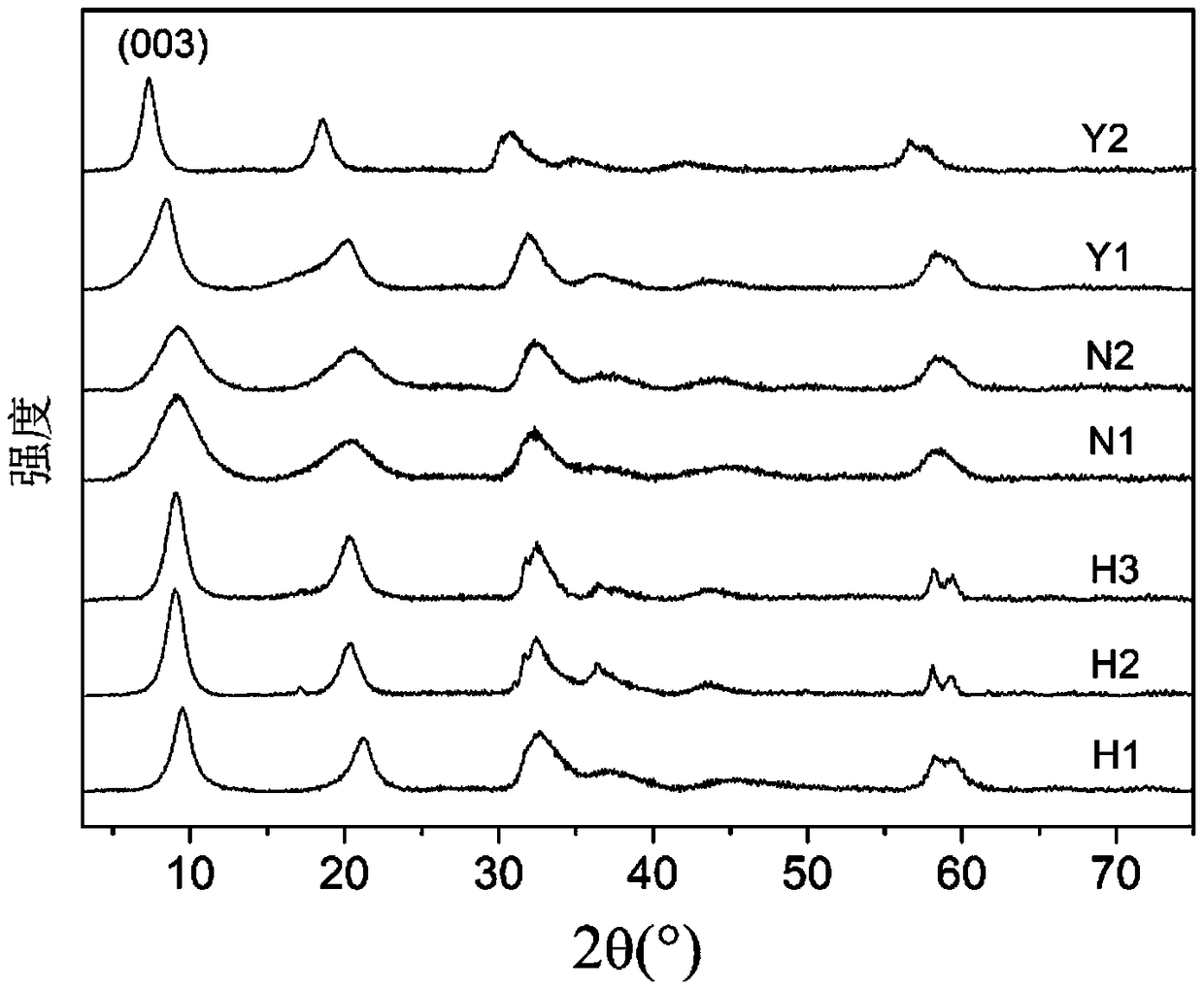
[0030] Prepare mixed solution A of 1mol / L cobalt nitrate and 0.2mol / L ammonium nitrate, prepare 0.1mol / LH 2 o 2 and 0.1mol / L sodium oxalate mixed solution B. Using the co-precipitation method, the A and B solutions were added to the 100mL sodium hydroxide bottom solution with an initial pH of 10 according to the flow rate of 6mL / min and 5mL / min, and the H was controlled during the process. 2 o 2 The molar ratio with cobalt salt is 1:12. During the reaction process, 2mol / L sodium hydroxide solution is continuously added to maintain the pH value of the reaction solution at about 10; The temperature was controlled at 55°C. After the reaction was completed, it was centrifuged, washed with a large amount of deionized water until the filter cake was neutral, and finally dried in vacuum at 70°C. The obtained sample was named H1, which is a spherical Co(II)Co(III) hydrotalcite material. The spherical Co(II) Co(III) hydrotalcite material is a layered double hydroxyl metal hydroxide...
Embodiment 2
[0033] Prepare mixed solution A of 1mol / L cobalt nitrate and 0.2mol / L ammonium nitrate, prepare 0.1mol / LH 2 o 2 and 0.1mol / L sodium oxalate mixed solution B. Using the co-precipitation method, the A and B solutions were added to the 100mL sodium hydroxide bottom solution with an initial pH of 10 according to the flow rate of 6mL / min and 6.67mL / min, and the H was controlled during the process. 2 o 2 The molar ratio with cobalt salt is 1:9. During the reaction process, 2mol / L sodium hydroxide solution is continuously added to maintain the pH value of the reaction solution at about 10; The temperature was controlled at 55°C. After the reaction, it was centrifuged, washed with a large amount of deionized water until the filter cake was neutral, and finally dried in vacuum at 70°C. The obtained sample was named H2, which is a spherical Co(II)Co(III) hydrotalcite material. The spherical Co(II) Co(III) hydrotalcite material is a layered double hydroxyl metal hydroxide formed by d...
Embodiment 3
[0036] Prepare mixed solution A of 1mol / L cobalt nitrate and 0.2mol / L ammonium nitrate, prepare 0.1mol / LH 2 o 2 and 0.1mol / L sodium oxalate mixed solution B. Using the co-precipitation method, the A and B solutions were added to the 100mL sodium hydroxide bottom solution with an initial pH of 10 according to the flow rate of 6mL / min and 10mL / min, and the H was controlled during the process. 2 o 2 The molar ratio with cobalt salt is 1:6. During the reaction process, 2mol / L sodium hydroxide solution is continuously added to maintain the pH value of the reaction solution at about 10; The temperature was controlled at 55°C. After the reaction, it was centrifuged, washed with a large amount of deionized water until the filter cake was neutral, and finally dried in vacuum at 70°C. The obtained sample was named H3, which is a spherical Co(II)Co(III) hydrotalcite material. The spherical Co(II) Co(III) hydrotalcite material is a layered double hydroxyl metal hydroxide formed by div...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com