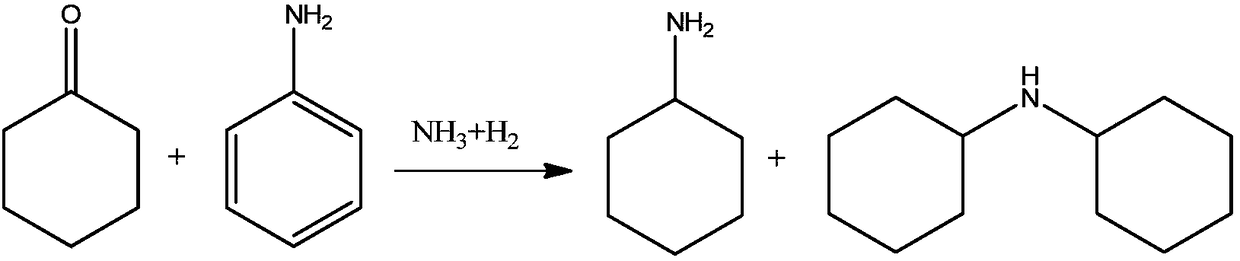
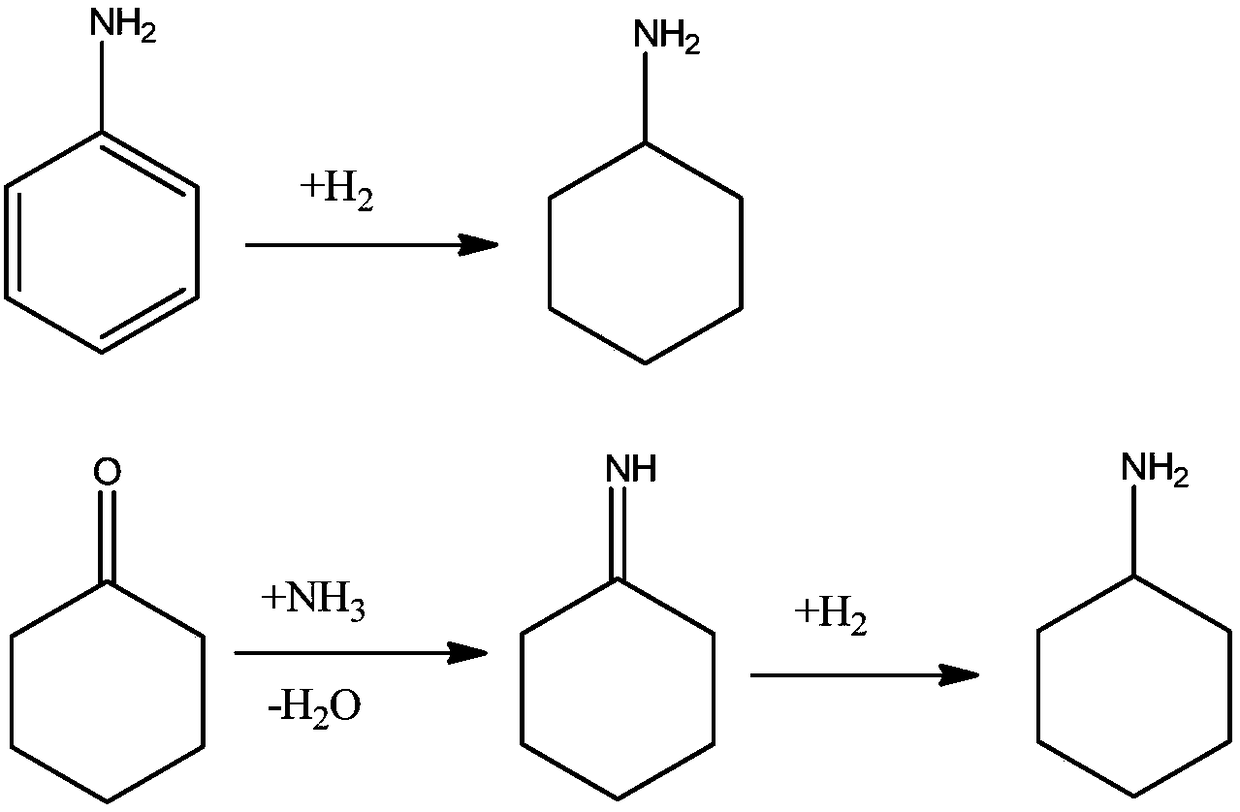
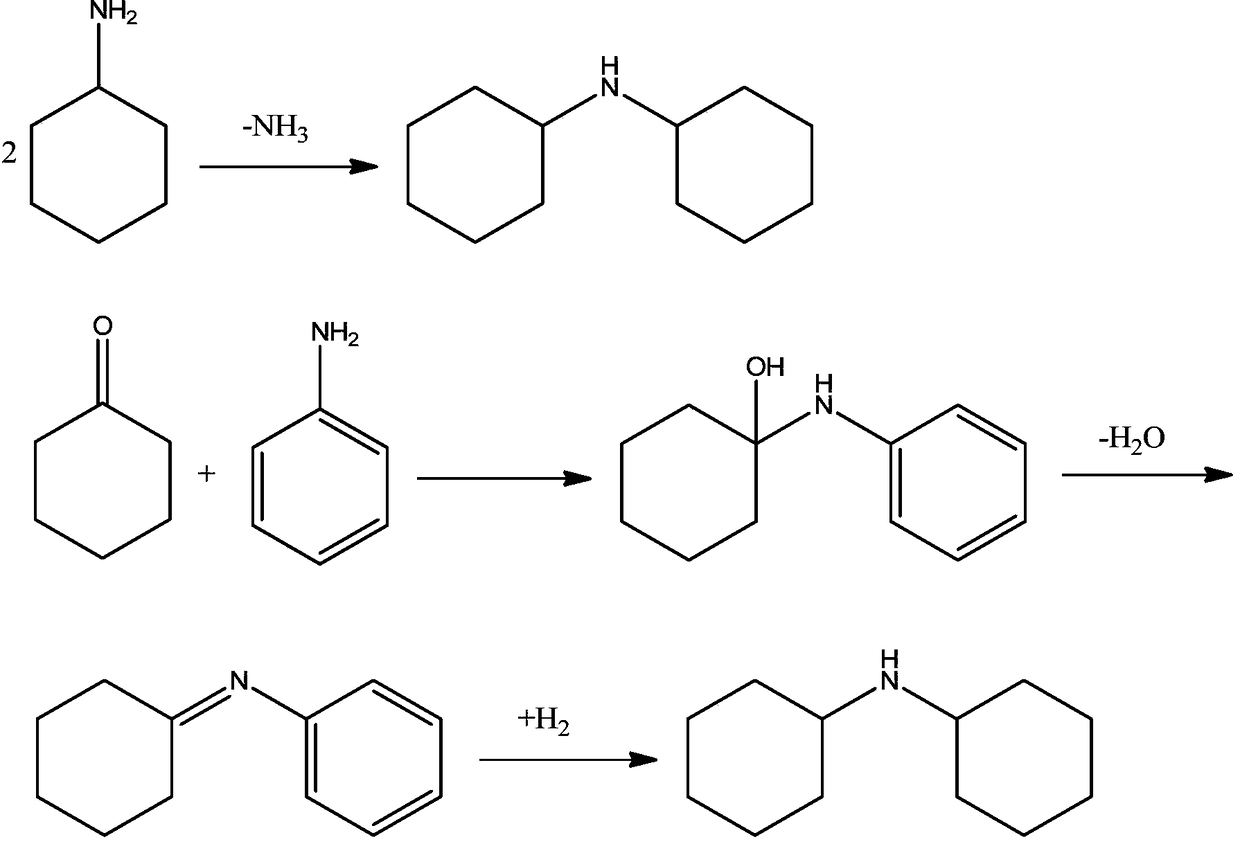
Joint production method for cyclohexylamine and dicyclohexylamine and catalyst system applied to method
A dicyclohexylamine and catalyst technology, applied in the field of organic compound preparation, can solve the problems of increasing the difficulty of industrialization, high labor intensity, and low purity of dicyclohexylamine rectification, so as to improve operation safety and reduce equipment cost. investment, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1# Preparation of supported Rh-Ni catalyst:
[0044] The spherical γ-Al 2 O 3 (Particle size 3mm, specific surface area 300m 2 / g) Calcination at 450°C for 4h. Use 100ml deionized water to prepare a nitrate impregnation solution containing 3g Rh, 5g Ni, 1g Re, 0.3g Zn, heat to 80℃ to form a homogeneous solution, and then add 90.7g γ-Al 2 O 3 The carrier is rotated and immersed in a water bath at 80°C for 4 hours, and then the water is gradually evaporated to dryness, and then baked in an oven at 120°C for 12 hours; finally moved to a muffle furnace, heated at 2-3°C / min to 500°C in an air atmosphere, and baked 6 After hours, the catalyst can be obtained after natural cooling. The composition of the catalyst is: Rh is 3wt%, Ni is 5wt%, Re is 1wt%, Zn is 0.3wt%, and the rest is γ-Al 2 O 3 , Based on the corresponding metal elements in the total mass of the catalyst.
Embodiment 2
[0046] 2# Preparation of supported Rh-Co catalyst:
[0047] The spherical γ-Al 2 O 3 (Particle size 3mm, specific surface area 300m 2 / g) Calcination at 450°C for 4h. Use 150ml deionized water to prepare a nitrate impregnation solution containing 3g Rh, 10g Co, 0.5g Re, 0.5g Mo, heat to 80℃ to form a homogeneous solution, and then add 86g γ-Al 2 O 3 The carrier is rotated and immersed in a water bath at 80°C for 4 hours, and then the water is gradually evaporated to dryness, and then baked in an oven at 120°C for 12 hours; finally moved to a muffle furnace, heated at 2-3°C / min to 500°C in an air atmosphere, and baked 6 After hours, the catalyst can be obtained after natural cooling. The composition of the catalyst is: Rh is 3wt%, Co is 10wt%, Re is 0.5wt%, Mo is 0.5wt%, and the rest is γ-Al 2 O 3 , Based on the corresponding metal elements in the total mass of the catalyst.
Embodiment 3
[0049] 3# Preparation of supported Rh-Ni catalyst:
[0050] The spherical silica (particle size 3mm, specific surface area 240m 2 / g) Calcination at 450°C for 4h. Prepare a nitrate impregnation solution containing 2g Rh, 10g Ni, 0.75g Re, 0.5g Zn with 150ml deionized water, heat to 80℃ to form a homogeneous solution, then add 86.75g silica carrier and rotate in a water bath at 80℃ After being immersed for 4 hours, the water is gradually evaporated and dried in an oven at 120°C for 12 hours; finally moved to a muffle furnace, heated at 2-3°C / min to 550°C for 8 hours in an air atmosphere, and the catalyst can be obtained after natural cooling . The composition of the catalyst is: Rh is 2wt%, Ni is 10wt%, Re is 0.75wt%, Zn is 0.5wt%, and the rest is silica, based on the corresponding metal elements in the total mass of the catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com