New application of asiaticoside in the preparation of medicines for treating skin cancer
A technology of asiaticoside and skin cancer, applied in the field of medicine, can solve the problems of no asiaticoside and poor treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 asiaticoside ointment
[0024] This embodiment provides a kind of cream for external use of 4% (W / W) asiaticoside, made from the following raw material components:
[0025] 400 parts by weight of asiaticoside, 1000 parts by weight of stearic acid, 200 parts by weight of glyceryl stearate, 200 parts by weight of beeswax, 600 parts by weight of palm oil, 600 parts by weight of butanediol, 200 parts by weight of ethanol, and 4800 parts by weight of water Parts, 3 parts by weight of carbomer, 2 parts by weight of triethanolamine.
[0026] According to above-mentioned raw material component, prepare Chinese medicine external cream of the present invention:
[0027] (1) Weigh stearic acid, glyceryl stearate, beeswax, and palm oil in proportion, heat in a water bath to melt, filter, and store at a constant temperature of 70-80°C;
[0028] (2) Weigh butanediol, ethanol, water, carbomer, and triethanolamine in proportion and add them to the abov...
Embodiment 2
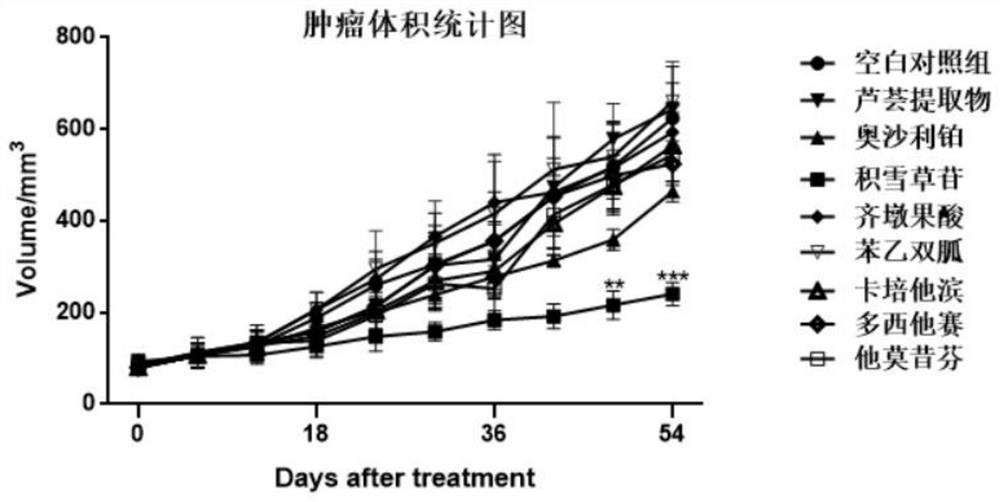
[0030] Example 2 The inhibitory effect of asiaticoside on mouse skin orthotopic tumors
[0031] 1. Experimental animals
[0032] SPF grade ICR mice, male, 6 weeks old, weighing 16-18 g, were purchased from Qinglongshan Experimental Animal Breeding Center. Raise in single cages, provide sufficient feed and drinking water, maintain a circadian rhythm of 12 hours of light and 12 hours of darkness, temperature 25±2°C, humidity 50%-70%, and the experimental process follows animal ethics.
[0033] 2. Main reagents
[0034] 7,12-Dimethylbenzanthracene (DMBA): Sigma, USA.
[0035] Phorbol ester (TPA): Sigma, USA.
[0036] Acetone: Sinopharm Chemical Reagent Co., Ltd.
[0037] Normal saline: Anhui Double Crane Pharmaceutical Co., Ltd.
[0038] Chloral hydrate: Shanghai Lingfeng Chemical Reagent Co., Ltd.
[0039] Capecitabine, oleanolic acid, phenformin, tamoxifen, docetaxel, oxaliplatin: Dalian Meilun Biotechnology Co., Ltd.
[0040] Prepared with reference to the method of Exa...
Embodiment 3
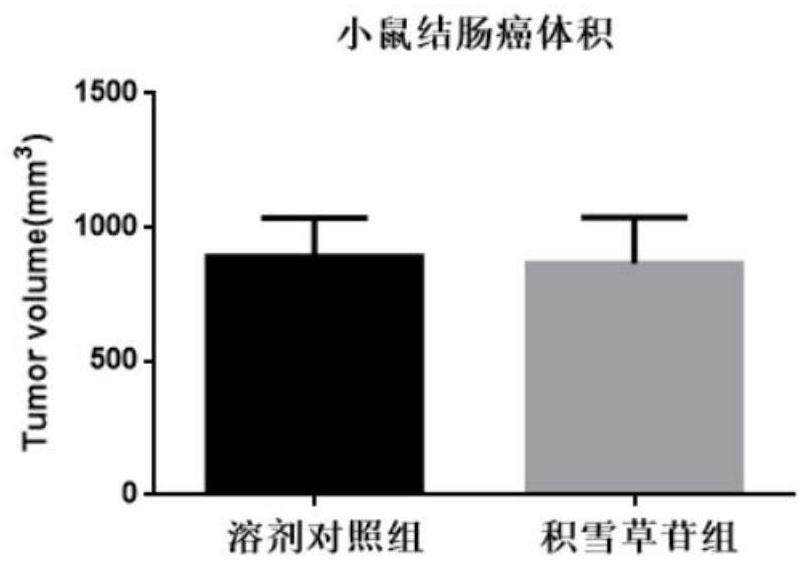
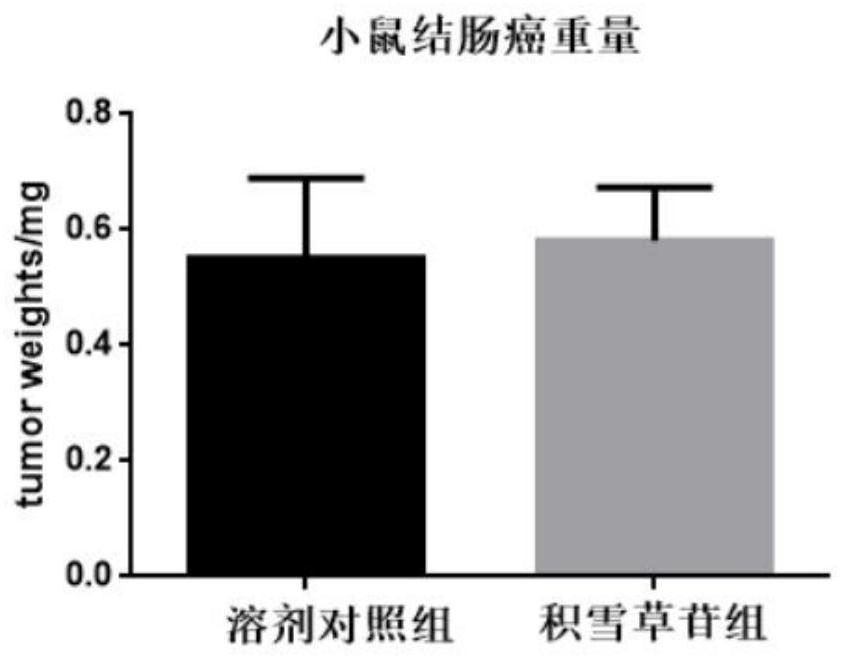
[0068] Example 3 The inhibitory effect of asiaticoside on colon cancer in mice
[0069] 1. Experimental animals: inbred NOD / SCID mice, female, 6 weeks old, weighing 16-18g, purchased from Beijing Weitong Lihua. Raise in single cages, provide sufficient feed and drinking water, maintain a circadian rhythm of 12 hours of light and 12 hours of darkness, temperature 25±2°C, humidity 50%-70%, and the experimental process follows animal ethics.
[0070] 2. Experimental process
[0071] (1) After the human colon cancer cell line HT29 cells were resuscitated and passaged, when the cells reached a stable growth state, they entered the next step of the experiment.
[0072] (2) Preparation of cell inoculum
[0073] Aspirate the cell supernatant, transfer the cells to a centrifuge tube, and centrifuge at 1000 rpm for 5 minutes; remove the supernatant, resuspend the cell mass with PBS, count, and adjust the cell density to 1×10 7 cells / mL, mix the cells evenly, take 100 μL of the cell s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com