Metal hematoporphyrin diether/diester compound, catalyst as well as preparation method thereof and cyclohexane catalytic oxidation method
A porphyrin diether, catalytic oxidation technology, applied in the direction of carbon-based compound preparation, organic compound preparation, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of high catalytic cost, low catalytic efficiency, and catalyst consumption Large and other problems, to achieve high catalytic efficiency, small dosage, and improve selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The third aspect of the present invention provides a kind of preparation method of above-mentioned catalyst, comprises the following steps:
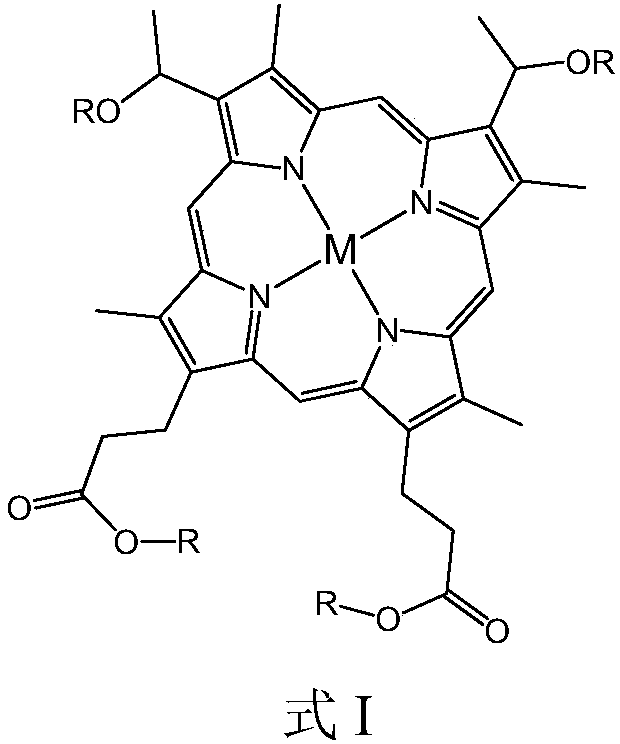
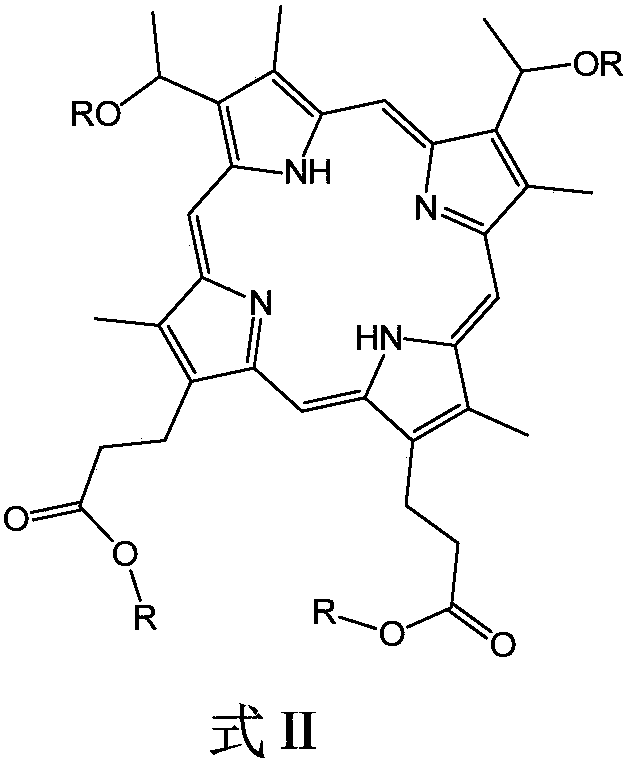
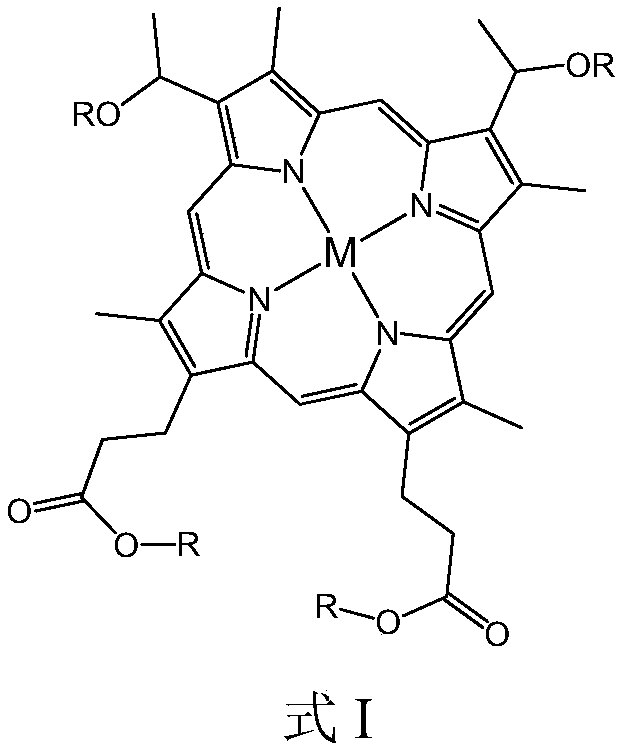
[0045] After hemin and saturated hydrogen bromide glacial acetic acid solution are mixed and reacted for a predetermined period of time, an alcohol with 6 to 21 carbon atoms, preferably a straight chain or branched chain fatty alcohol, is added and reacted under ultrasonic vibration conditions to obtain the structural formula: Compound shown in formula II. The reaction temperature is preferably normal temperature, such as 20°C to 30°C, and the predetermined time is preferably 12 hours to 24 hours.
[0046]
[0047] Reacting Fe salt, Co salt and / or Ni salt with the compound represented by formula II in a solvent at a temperature of 130° C. to 150° C. to obtain the metallohaematoporphyrin diether diester compound. The reaction time may preferably be 3 to 5 hours, and the solvent may be, for example, dimethylformamide (DMF). The...
Embodiment 1
[0067] 300g of cyclohexane was added to the reactor, and then the metallohaematoporphyrin diether diester compound a (see Table 1) with a mass concentration of 1.0ppm was added, and the reactor was sealed. Feed nitrogen to replace, and when the oxygen content in the tail gas is lower than 0.5%, close the tail gas valve, continue to feed nitrogen, make the pressure in the still reach 0.2MPa, and seal the reaction kettle. Turn on the heating system and stir, heat the reaction system to 110°C, then feed air, open the tail gas valve, make the reaction system pressure reach 0.65MPa, and start timing. After reaction advances 50min, stop feeding air, cooling down, reaction system is analyzed, catalytic oxidation analysis result is as shown in table 2.
Embodiment 2-9
[0069] The difference of embodiment 2-9 and the hexanaphthene catalytic oxidation method of embodiment 1 is that the central metal atom M and alkyl R of the catalyst b-i adopted in embodiment 2-9 are different from the catalyst (a ). The catalytic oxidation analysis results of Examples 2-9 are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com