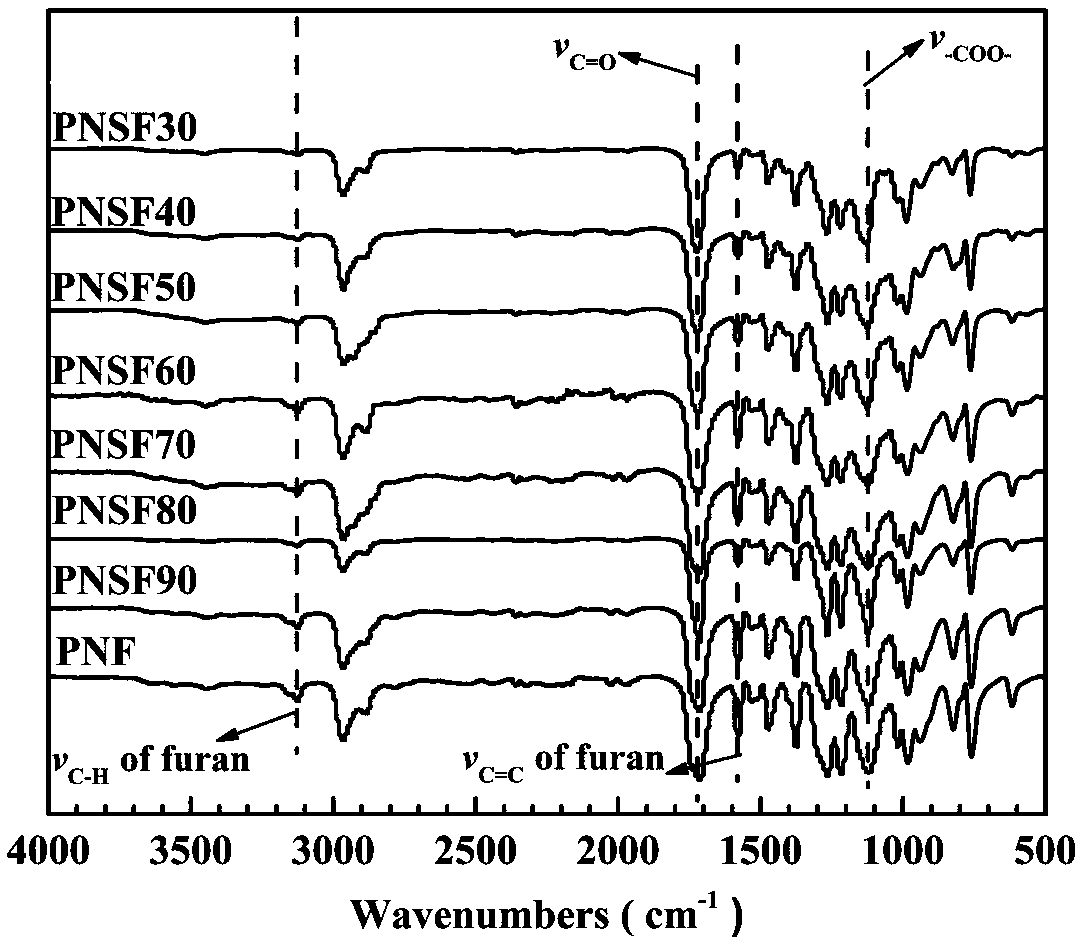
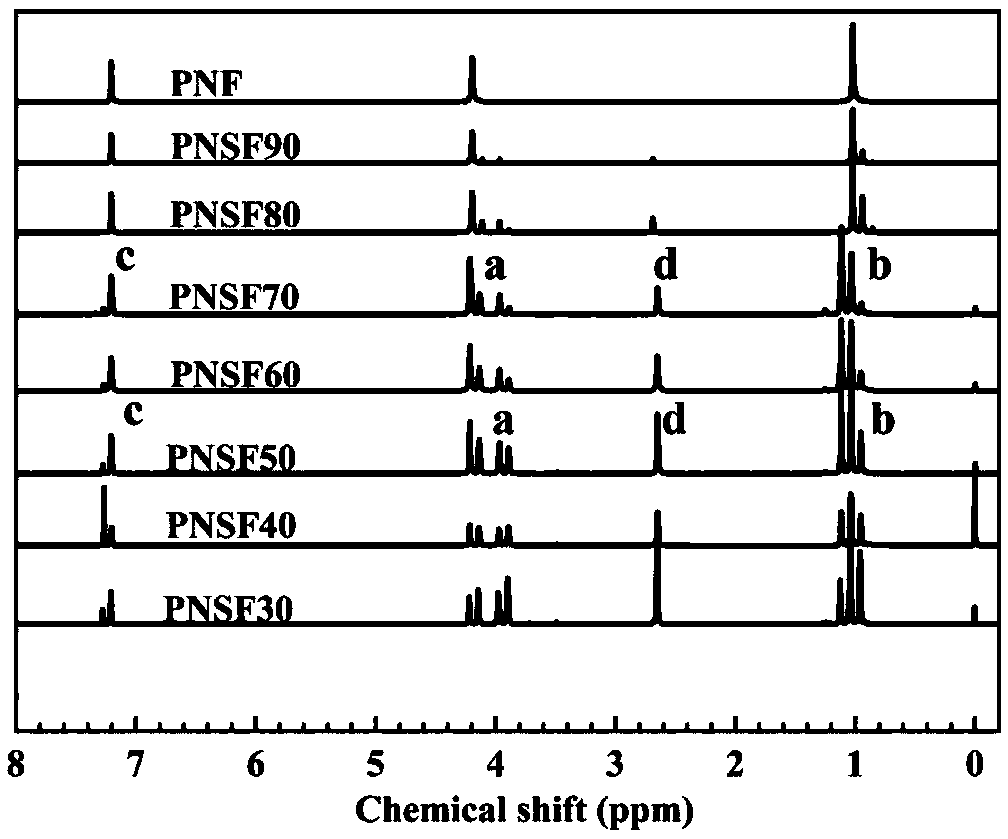
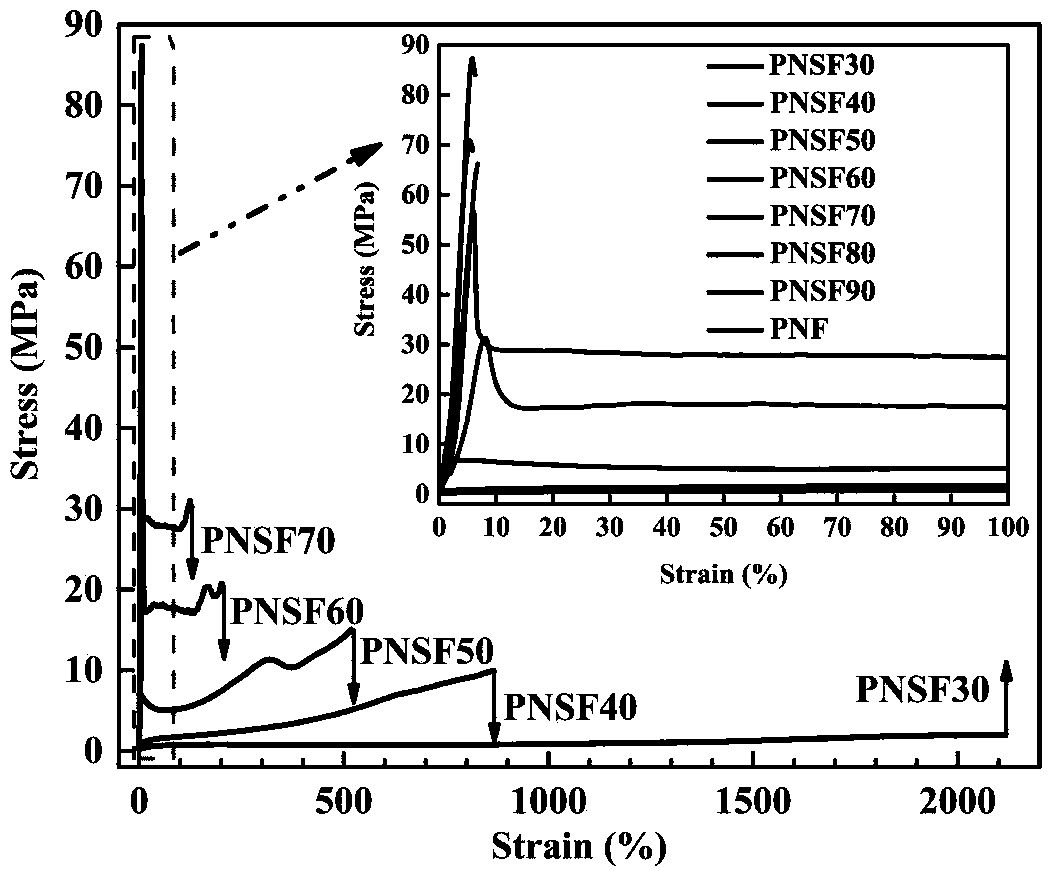
Poly(neopentyl glycol 2,5-furandicarboxylate-1,4-succinate) and preparation method and article thereof
A technology of dimethyl furandicarboxylate and furandicarboxylate is applied in the field of poly-2,5-furandicarboxylate-1,4-succinate neopentyl glycol and its preparation, and can solve the problems of brittleness and insufficient barrier properties , insufficient thermal stability, etc., to achieve the effect of being controllable, promoting sustainable development and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of poly-2,5-furandicarboxylate-1,4-butanedioic acid neopentyl glycol ester provided by the invention comprises:
[0039] S1, mixing 2,5-furandicarboxylic acid or its diester, 1,4-butanedioic acid or its diester, neopentyl glycol and a catalyst, and performing an esterification reaction to obtain product A and product B;
[0040] The structural formula of the product A is shown in the following formula (2):
[0041]
[0042] The structural formula of the product B is shown in the following formula (3):
[0043]
[0044] S2, subjecting the product A and the product B to a polycondensation reaction to obtain poly-2,5-furandicarboxylic acid-1,4-butanedioic acid neopentyl glycol ester.
[0045]In step S1, the molar ratio of the 2,5-furandicarboxylic acid or its diester to 1,4-butanedioic acid or its diester is 1:9 to 9:1, and the 2,5-furandicarboxylic acid di The molar ratio of the sum of methyl ester or its diester and 1,4-butanedioic acid or ...
Embodiment 1
[0068] In the poly-2,5-furandicarboxylic acid-1,4-succinate neopentyl glycol ester of this embodiment, m:n=6:4, which is defined as PNSF40, and the specific preparation process is as follows:
[0069] (1) Esterification reaction: Dimethyl 2,5-furandicarboxylate, dimethyl 1,4-succinate, new Pentylene glycol, tetrabutyl titanate, and zinc acetate were added to the reactor. Under the protection of high-purity nitrogen, react at 180° C. for 5 h until the content of methanol produced exceeds 95% of the theoretical value, and intermediate products including product A and product B are obtained.
[0070] (2) Polycondensation reaction: continue to add 10 mg of antimony trioxide and 15 mg of heat stabilizer triphenyl phosphate into the reactor. Then reduce the pressure to 1000Pa, and then reduce the pressure to less than 20Pa after 30min, and react at 200°C for 20h to obtain a light yellow PNSF40 product.
[0071] NMR, infrared, DSC, TGA, mechanical properties, barrier properties, de...
Embodiment 2
[0079] In the poly-2,5-furandicarboxylic acid-1,4-succinate neopentyl glycol ester of this embodiment, m:n=5:5, which is defined as PNSF50, and the specific preparation process is as follows:
[0080] (1) Esterification reaction: Dimethyl 2,5-furandicarboxylate, dimethyl 1,4-succinate, neopentyl glycol with molar amounts of 0.05 moles, 0.05 moles, 0.25 moles and 10 mg respectively , Zinc acetate was added to the reactor. Under the protection of high-purity nitrogen, react at 200° C. for 2 h until the content of methanol produced exceeds 95% of the theoretical value, and intermediate products including product A and product B are obtained.
[0081] (2) Polycondensation reaction: Continue to add 15 mg of isopropyl titanate and 15 mg of heat stabilizer triphenyl phosphate to the reactor, then reduce the pressure to 1000 Pa, then reduce the pressure to less than 20 Pa after 30 minutes, and react at 260 ° C for 3 hours , a pale yellow PNSF50 product was obtained.
[0082] NMR, in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com