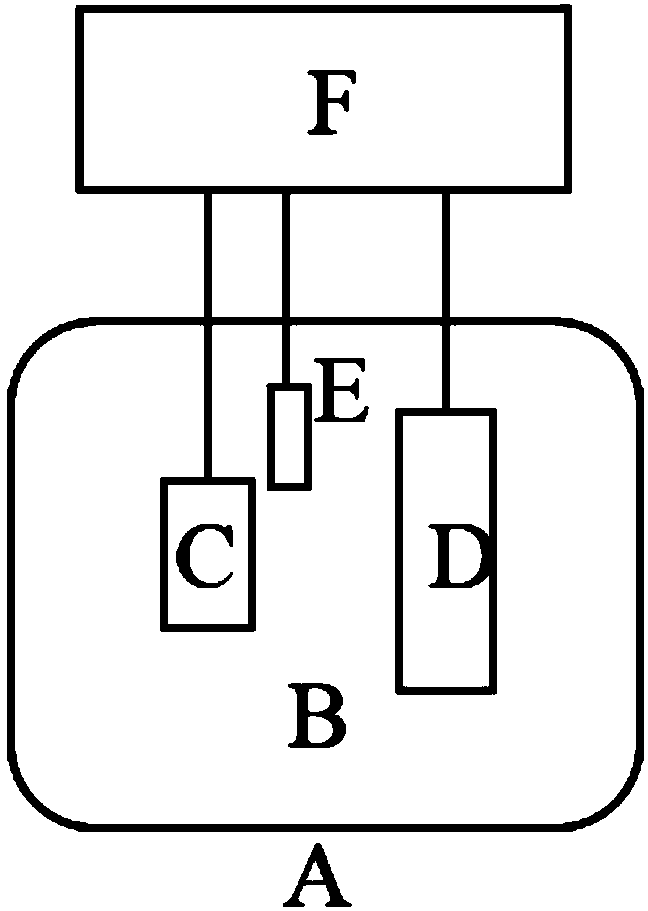
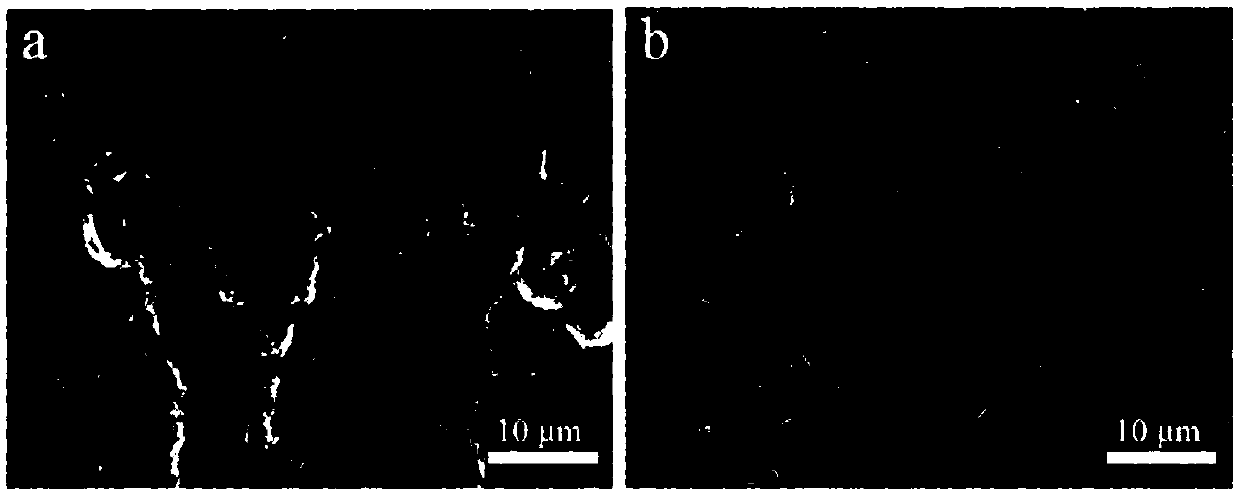
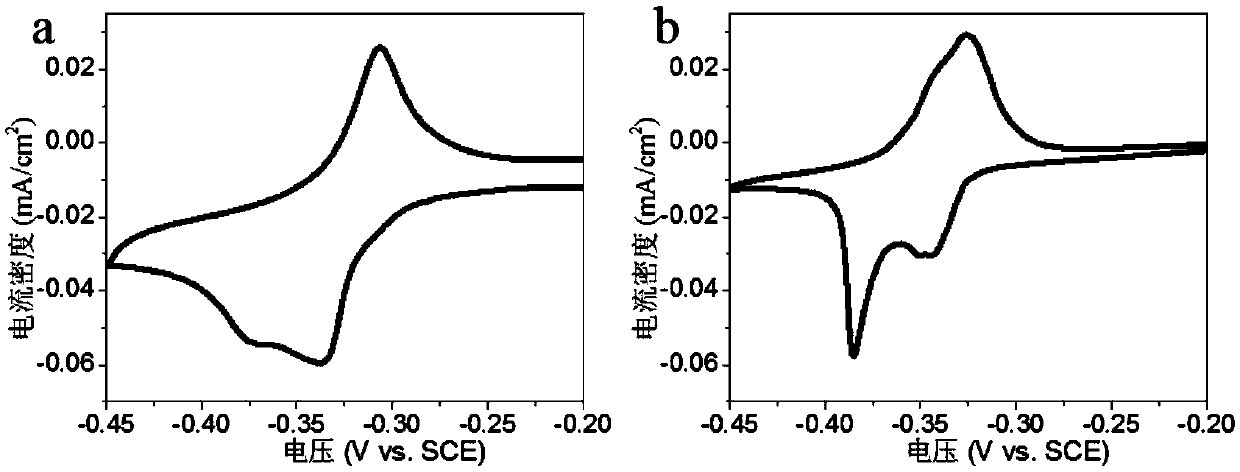
Metal lithium support and preparation method and application thereof
A technology of support and metal lithium, applied in the field of electrochemistry, can solve the problems of ignoring the use of electrochemically active space, ignoring the influence of affinity nucleation, the growth process, and the inability of the current collector to take advantage of the high specific surface, so as to improve the Safety and operability, uniform and stable electrodeposition, and the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of the metal lithium support is carried out as follows:
[0037] 1) Put a metal copper or metal nickel support into the electrochemical cell as a working electrode; inject an electropolishing liquid into the electrochemical cell (usually electropolishing liquids of metal copper and metal nickel can be used in the present invention), Electrode applies 1.0~2.0V anode potential or 100~300mA / cm 2 The anode current makes the metal dissolution reaction occur, and the reaction time is 15-60s.
[0038] 2) After the reaction is completed, take out the support on the working electrode and rinse it with secondary water, put it into the electrochemical cell again as the working electrode, and replace the electropolishing liquid in the electrochemical cell with the crystal face seal containing 1-20 mM. 0.5~2.0M soluble metal copper or nickel salt electrolyte of capping agent; apply -0.2~-0.05V cathode potential or -0.1~-0.05mA / cm to the above working electrode 2 The catho...
Embodiment 2
[0041] The difference between this embodiment and embodiment 1 is that in step 1), copper foil is used as the working electrode, and an anode potential of 2.0V is applied to the working electrode to cause the metal to dissolve out, and the reaction time is 15s. Others are the same as in Example 1.
Embodiment 3
[0043] The difference between this embodiment and embodiment 1 is that in step 1), a copper mesh is used as the working electrode, and an anode potential of 1.0V is applied to the working electrode to cause the metal to dissolve out, and the reaction time is 60s. Others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com