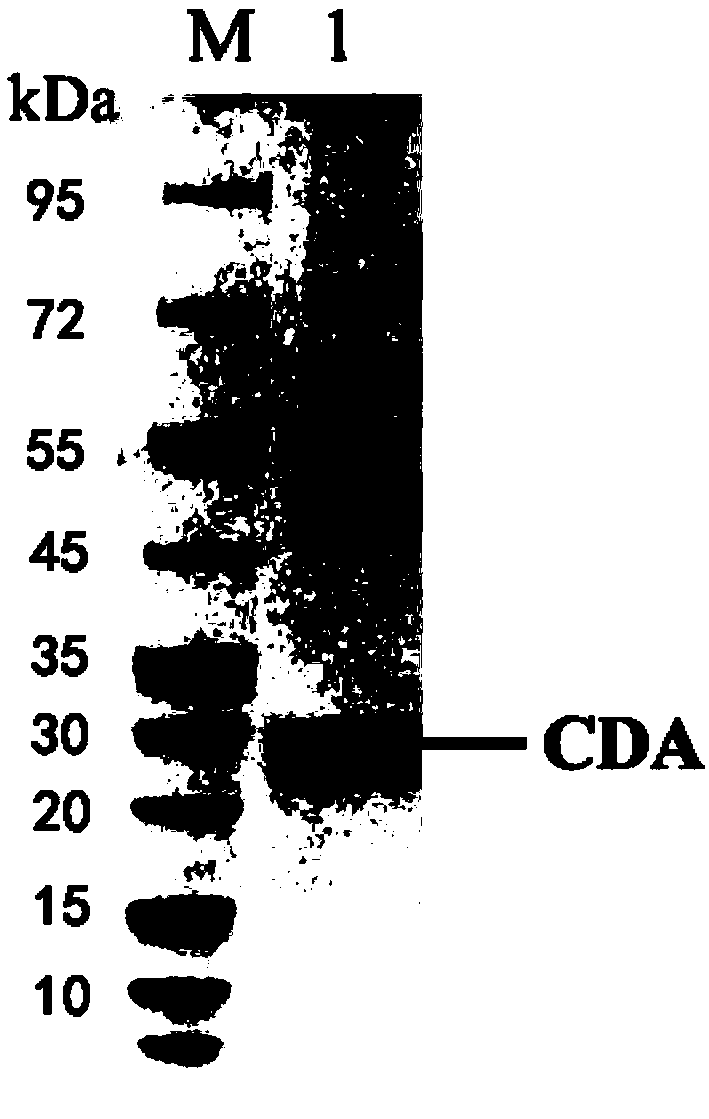
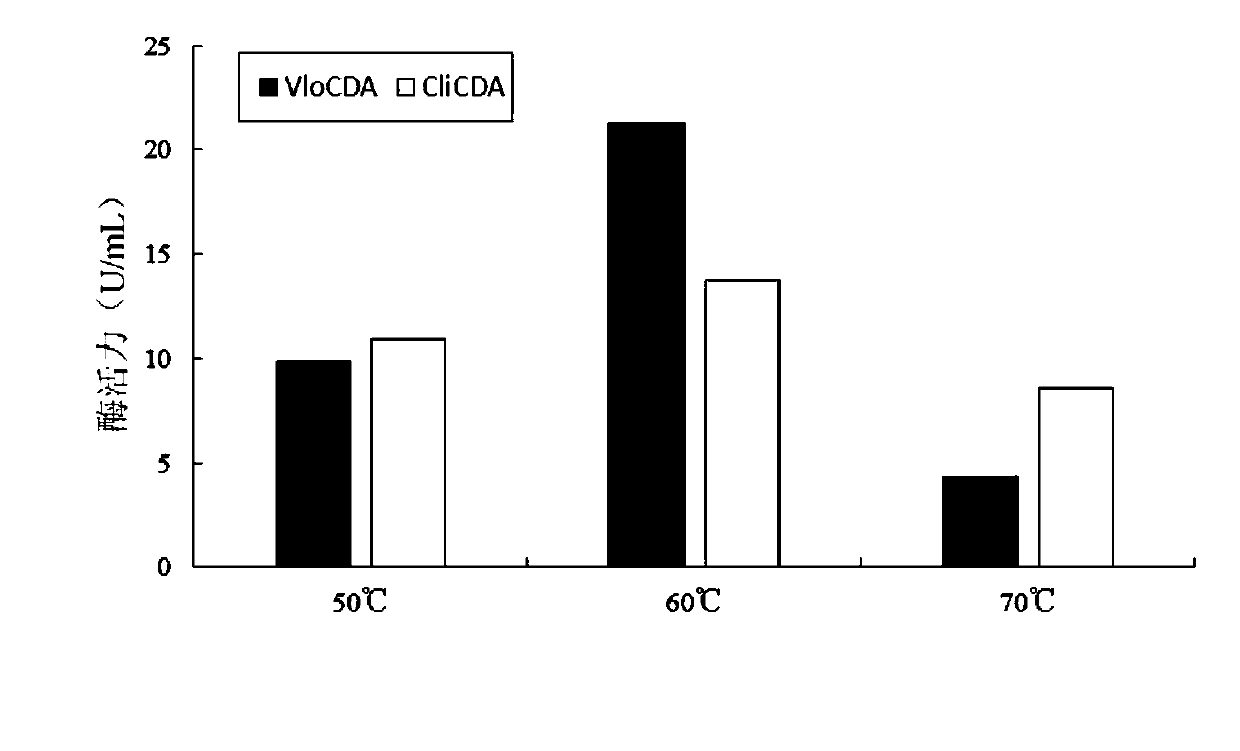
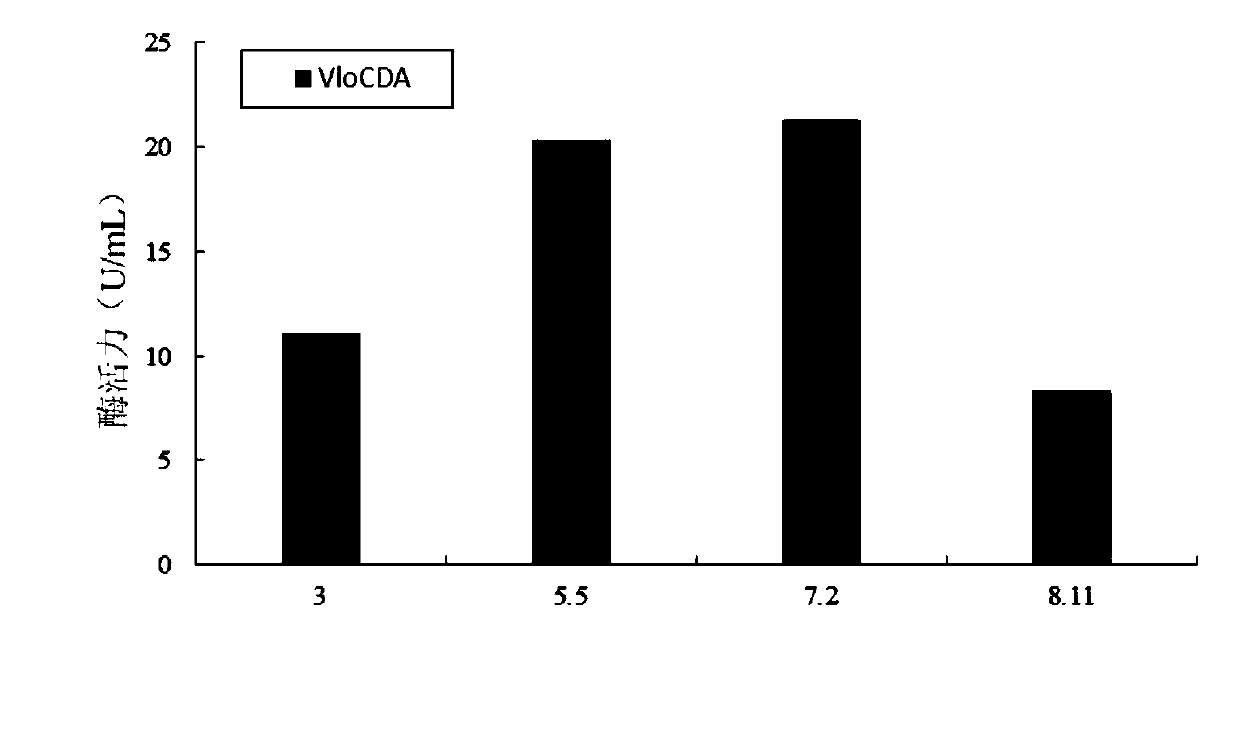
Chitin deacetylase and application thereof
A technology of vegetable deacetylase and vegetable deacetylase, which is applied in the field of chitin deacetylase, can solve the problems of optimal temperature difference, inadaptability to industrial material reaction conditions, etc., achieve low production conditions, reduce production costs, and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0043] According to a preferred embodiment of the present invention, Escherichia coli transformed cells or Bacillus subtilis transformed cells can be obtained by the following method: using chemical transformation method, the expression vector of the present invention is transformed into Escherichia coli competent cells or Bacillus subtilis competent cells cells; then the bacterial suspension was spread on the plate and cultured until a single colony appeared.
[0044] Five. The test kit of the present invention
[0045] The kit of the present invention is a kit comprising one or more of the chitin deacetylase of the present invention, the DNA molecule encoding the chitin deacetylase of the present invention, the expression vector of the present invention and the transformed cell of the present invention .
[0046] The kit can comprise a container and a label or package insert on or associated with the container. Suitable containers include, for example, bottles, vials, syri...
Embodiment 1
[0058] Design and screening of embodiment 1 chitin deacetylase
[0059] Searching for potential thermostable sugar isomerases in the NCBI database, and after sequence alignment analysis and strain source investigation, an unknown protein from Verticillium dahliae Vlo was found, whose amino acid sequence is shown in SEQ ID NO: 1 As shown, it is speculated that it has deacetylase activity.
[0060] Furthermore, codon optimization is performed on the coding sequence of the amino acid sequence to obtain the nucleotide sequence shown in SEQ ID NO:2.
Embodiment 2
[0061] Example 2 Expression of chitin deacetylase in Escherichia coli
[0062] The nucleotide sequence shown in SEQ ID NO: 2 was synthesized by General Biosystems (Anhui) Co., Ltd., a BamH I restriction site was added at its 5' end, and a hexahistidine tag was added before the stop codon. coding sequence, and add Pst I and Not I restriction sites at the 3' end. The synthesized fragment was double-digested by BamH I and Not I (New England Biolabs, NEB), and the double-digested fragment was ligated with T4 DNA ligase (Takara) to a DNA that was also double-digested by BamH I and NotI pET24a(+) vector (General Biosystems (Anhui) Co., Ltd.). The ligation product was transformed into Escherichia coli DH5α competent cells (Beijing Quanshijin Biotechnology Co., Ltd.), cultured on LB solid plates with kanamycin, and positive clones were screened. Pick a single colony for colony PCR verification; and perform sequencing verification for clones that are positive for colony PCR.
[0063...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com