Preparation method for 1, 2, 3, 4-cyclopentane tetracarboxylic acid dianhydride
A technology of cyclopentane tetracarboxylic acid and dicarboxylic anhydride, which is applied in the field of fine chemicals, and can solve the problems of violent exothermic reaction and runaway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
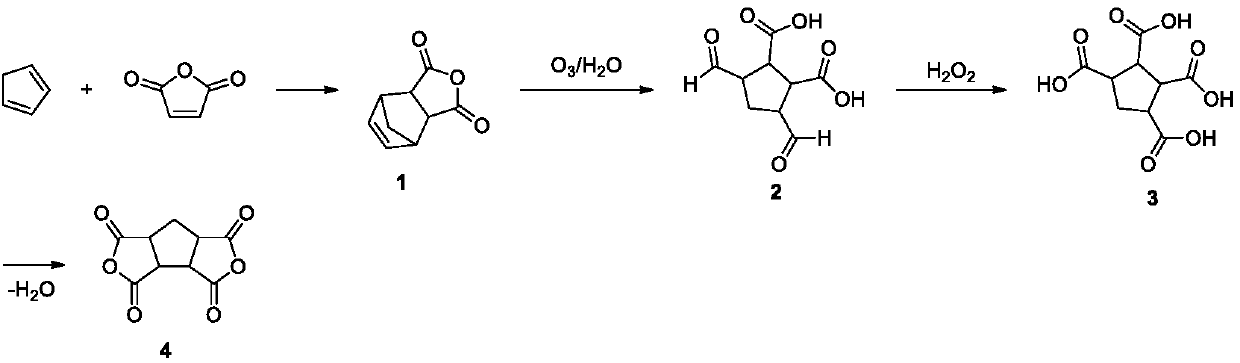
[0021] Put 98.00g (1.00mol) of maleic anhydride in the reactor, add 300.00g of ethyl acetate to dissolve, slowly add 72.71g (1.10mol) of cyclopentadiene dropwise at low temperature, control the reaction temperature -10°C, and react During the process, white crystals were continuously precipitated. After the cyclopentadiene was added dropwise, the reaction was completed. After filtration and vacuum drying, 157.60 g of white solids were obtained, with a yield of 96.10%.
[0022] Place 157.60g (0.96mol) of 5-norbornene-2,3-dicarboxylic acid anhydride in the bubble reactor, add 800.00g of acetic acid and 160.00g of distilled water to dissolve it, at low temperature, from the bubble reactor Ozone gas was continuously fed into the bottom, and the reaction temperature was controlled at 0°C. After 6 hours of reaction, TLC detected that the raw materials had reacted completely, and the reaction was stopped. The prepared crude product was directly carried out to the next step without tre...
Embodiment 2
[0026] Put 98.00g (1.00mol) of maleic anhydride in the reactor, add 300.00g of ethyl acetate to dissolve, slowly add 72.71g (1.10mol) of cyclopentadiene dropwise at low temperature, control the reaction temperature -0°C, and react During the process, white crystals were continuously precipitated. After the cyclopentadiene was added dropwise, the reaction was completed. After filtration and vacuum drying, 156.35 g of white solids were obtained, with a yield of 95.24%.
[0027] Put 156.35g (0.95mol) of 5-norbornene-2,3-dicarboxylic anhydride in the bubble reactor, add 700.00 formic acid and 150.00g of distilled water to dissolve it, at low temperature, from the bottom of the bubble reactor Ozone gas was continuously introduced, and the reaction temperature was controlled at 5°C. After 7 hours of reaction, TLC detected that the raw materials were completely reacted, and the reaction was stopped, and the prepared crude product was directly carried out to the next step without treat...
Embodiment 3
[0031] Put 98.00g (1.00mol) maleic anhydride in the reactor, add 300.00g ethyl acetate to dissolve, slowly add 72.71g (1.10mol) cyclopentadiene dropwise at low temperature, control the reaction temperature at 10°C, the reaction process White crystals were continuously precipitated, and the reaction ended after cyclopentadiene was added dropwise. After filtration and vacuum drying, 153.31 g of white solids were obtained, with a yield of 93.48%.
[0032] Put 153.31g (0.93mol) of 5-norbornene-2,3-dicarboxylic acid anhydride in the bubble reactor, add 600.00g of ethanol and 150.00g of distilled water to dissolve it, at low temperature, from the bottom of the bubble reactor Ozone gas was continuously introduced, and the reaction temperature was controlled at 10°C. After 7.5 hours of reaction, TLC detected that the raw materials had reacted completely, and the reaction was stopped, and the prepared crude product was directly carried out to the next step without treatment.
[0033] P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com