Myliataylori sesquiterpene synthetase MTa and gene sequence thereof
A technology of sesquiterpene synthase and moss moss, which is applied in genetic engineering, plant genetic improvement, lyase, etc., can solve the problems of rare plants of Caryophyllaceae and little research on moss plants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
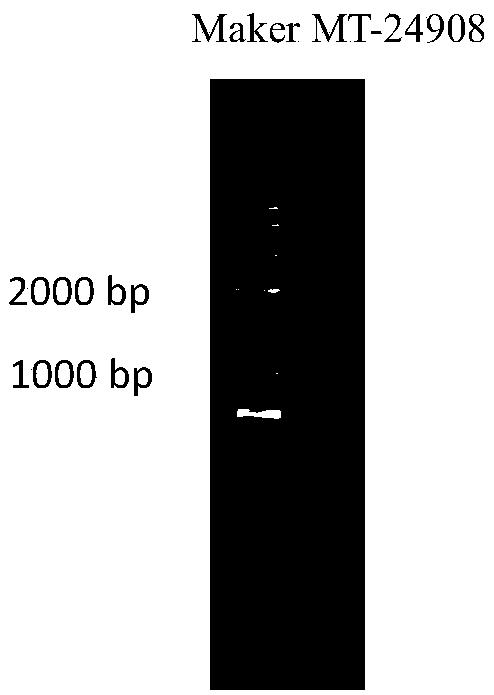
[0020] Example 1. Cloning and expression of the WQ011 / pESC-LEU-MTa bacterial strain Sesquiterpene synthase gene MT-24908
[0021] Total RNA was extracted from Lily calyx by RNAprep pure Plant Kit. The first-strand cDNA was synthesized using the Clontech SMARTer PCRcDNA Synthesis Kit, the first-strand cDNA was amplified by PCR to synthesize the second-strand cDNA, and then the double-strand DNA was amplified by secondary PCR and used for SMRTbell library construction, and PacBio ISO-Seq was used platform for sequencing.
[0022] According to the MT-24908 gene sequence SEQ ID NO.2 obtained by sequencing, primers were designed to amplify the target gene. The primer sequences are as follows:
[0023] Primer MT-24908-F: 5'CGC GGATCC ATGAATGCAGCAGGTGCATT 3' (SEQ ID NO. 3)
[0024] Primer MT-24908-R: 5'CCG CTCGAG TCATCGAGCCGATTGAGCCG 3' (SEQ ID NO. 4)
[0025] Using the cDNA sequence obtained by reverse transcription as a template, the MT-24908 gene sequence was amplified by P...
Embodiment 2
[0047] Example 2. Induced fermentation
[0048] (1) The transformant WQ011 / pESC-LEU-MTa was transferred to 50 mL of synthetic medium (with out Leu), 180 r / min, 30° C., and cultured on a shaking table for 30 h.
[0049] (2) At 30h, 10g / L galactose was added to induce expression to 90-120h, and at 36h and 72h, 10g / L ethanol was added as a supplementary carbon source.
[0050] (3) The fermentation broth was extracted with 3 mL of n-hexane for 15 min to detect by GC-MS.
Embodiment 3
[0051] Embodiment 3.GC-MS detects fermentation product
[0052] (1) GC-MS detection method
[0053] Chromatographic column: TG-5MS; Ion source; EI, 70eV; Injection volume: 1uL; Injection temperature: 200°C; Detector temperature: 280°C; Column temperature: 240°C; / min Raise the temperature to 280°C and maintain it for 5min.
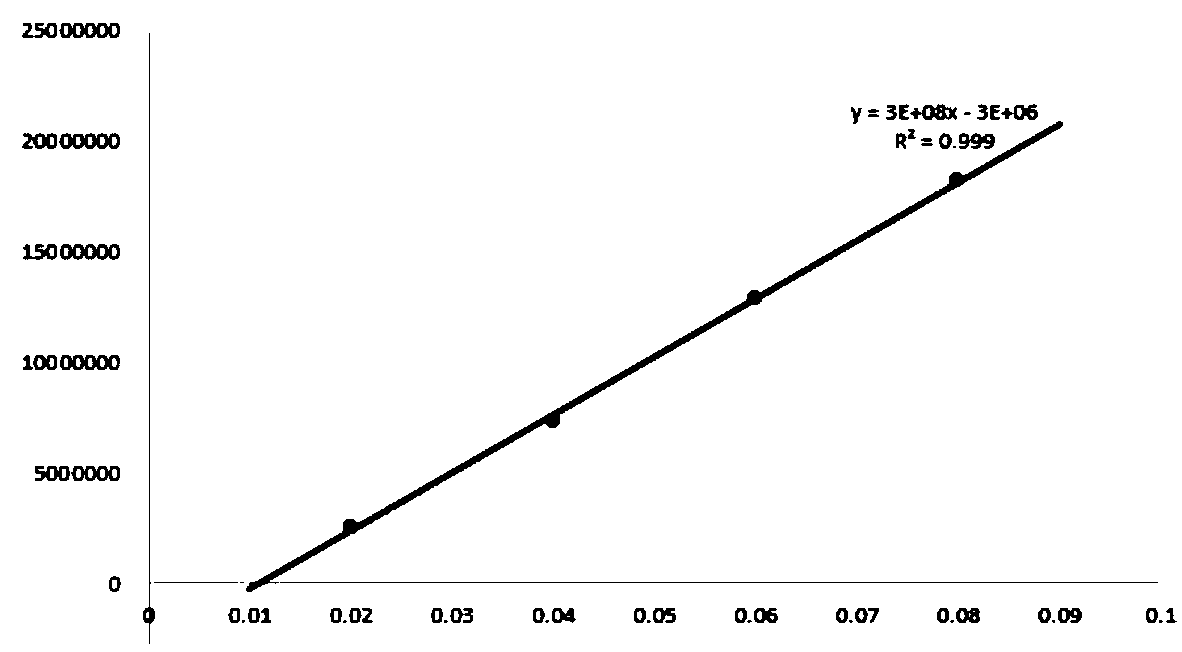
[0054] (2) The output was calculated by the external standard method. Accurately prepare 0.02, 0.04, 0.06, 0.08 and 1.0mg / L standard substances respectively, take the peak area as the ordinate, and the concentration as the abscissa to draw a standard curve (such as figure 2 shown).
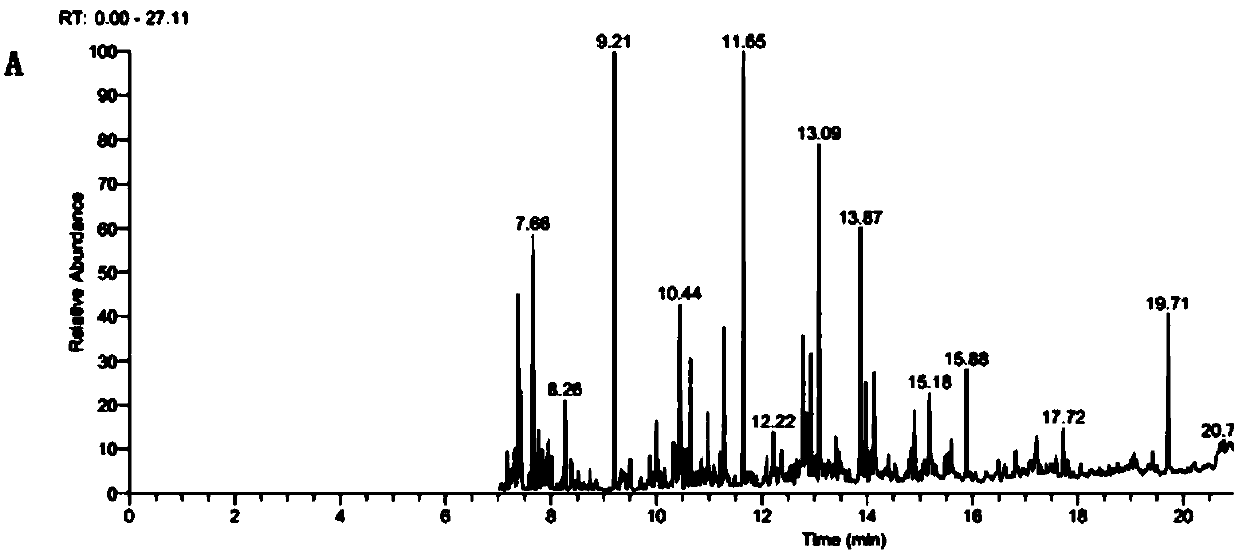
[0055] The results show that (such as image 3 Shown): The target peak in the GC-MS spectrum, compared with the reference mass spectrum data in the NIST14 database, it was found that the chromatographic peak of the n-hexane extract of the WQ011 / pESC-LEU-MTa strain fermentation broth at a retention time of 11.28min was orange For tertiary alcohol, the output of 50mL shake...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com