Triphenylamine substituted-vinyl modified phenanthroimidazole compound, preparation method thereof and application of compound as electroluminescent device
A technology of phenanthroimidazole and triphenylethylene, which is applied in the field of organic light-emitting materials and optoelectronic devices, can solve the problem of taking into account the good carrier transport ability of light-emitting materials, high fluorescence quantum yield, and weak electron transport and hole transport capabilities. , unbalanced device carrier transport and other issues, to achieve the effect of strengthening the aggregation-induced luminescence effect, carrier injection and transport balance, and avoiding efficiency decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
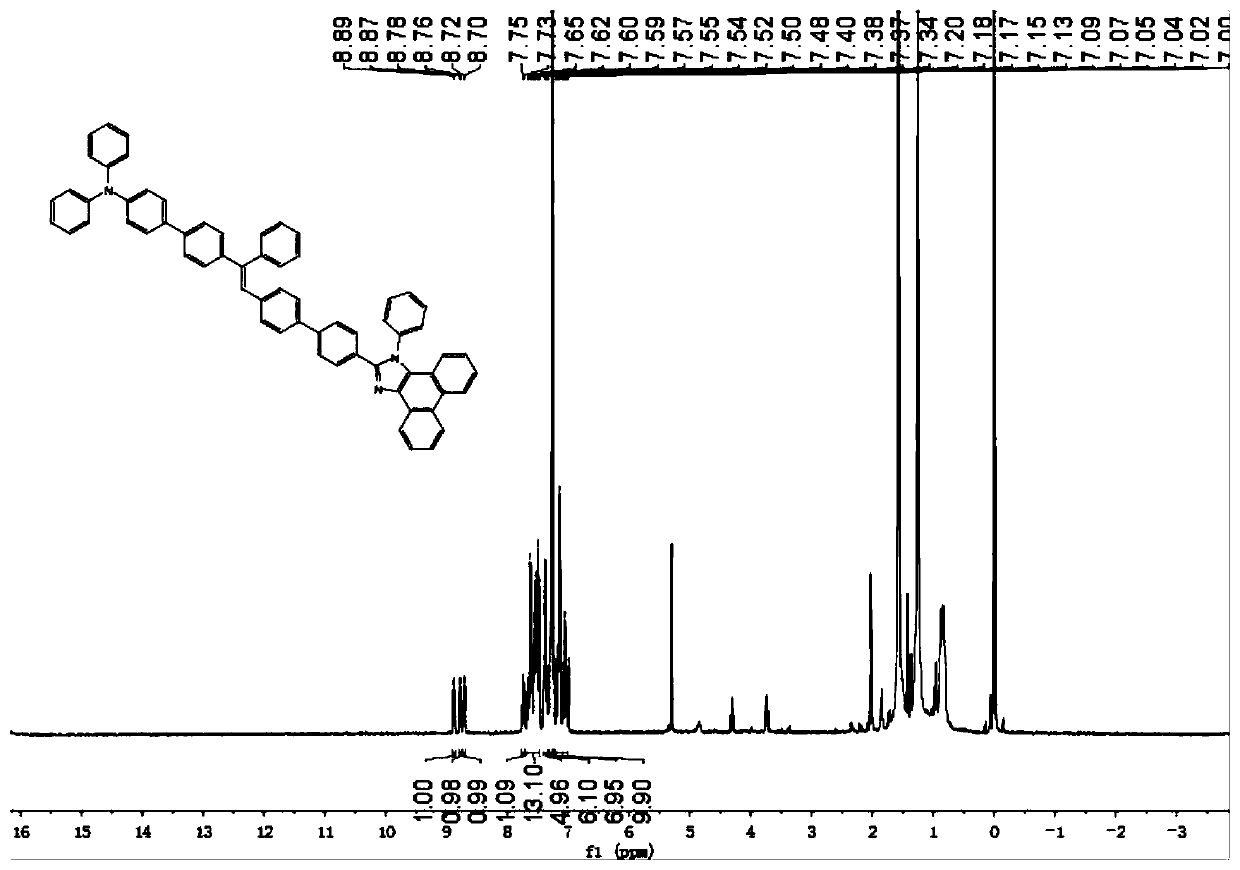
[0048] The preparation of the phenanthroimidazole that embodiment 1 triphenylamine replaces-tristyryl modification
[0049]Step 1: Preparation of 4-bromophenanthroimidazole (i.e. compound 1):
[0050] Add 4-bromobenzaldehyde (1.86g, 10mmol), aniline (1.49g, 10mmol), 9,10-phenanthrenequinone (2.08g, 10mmol), ammonium acetate (4.62g, 60mmol) into a 100ml two-necked flask in sequence, And 60ml of glacial acetic acid was added to obtain a dark brown suspension. After the mixture was stirred at 120° C. for 2 hours, the color of the solution changed from dark brown to black, and the reaction mixture was stirred overnight (12 hours) at room temperature. The crude product was isolated by washing with methanol and filtration, then dried in vacuo. Using silica gel powder as stationary phase, petroleum ether and dichloromethane as eluent (petroleum ether: CH 2 Cl 2 ,1:2) to obtain a white powder with a yield of 82.1%.
[0051] The reaction equation is as follows:
[0052]
[005...
Embodiment 2 3
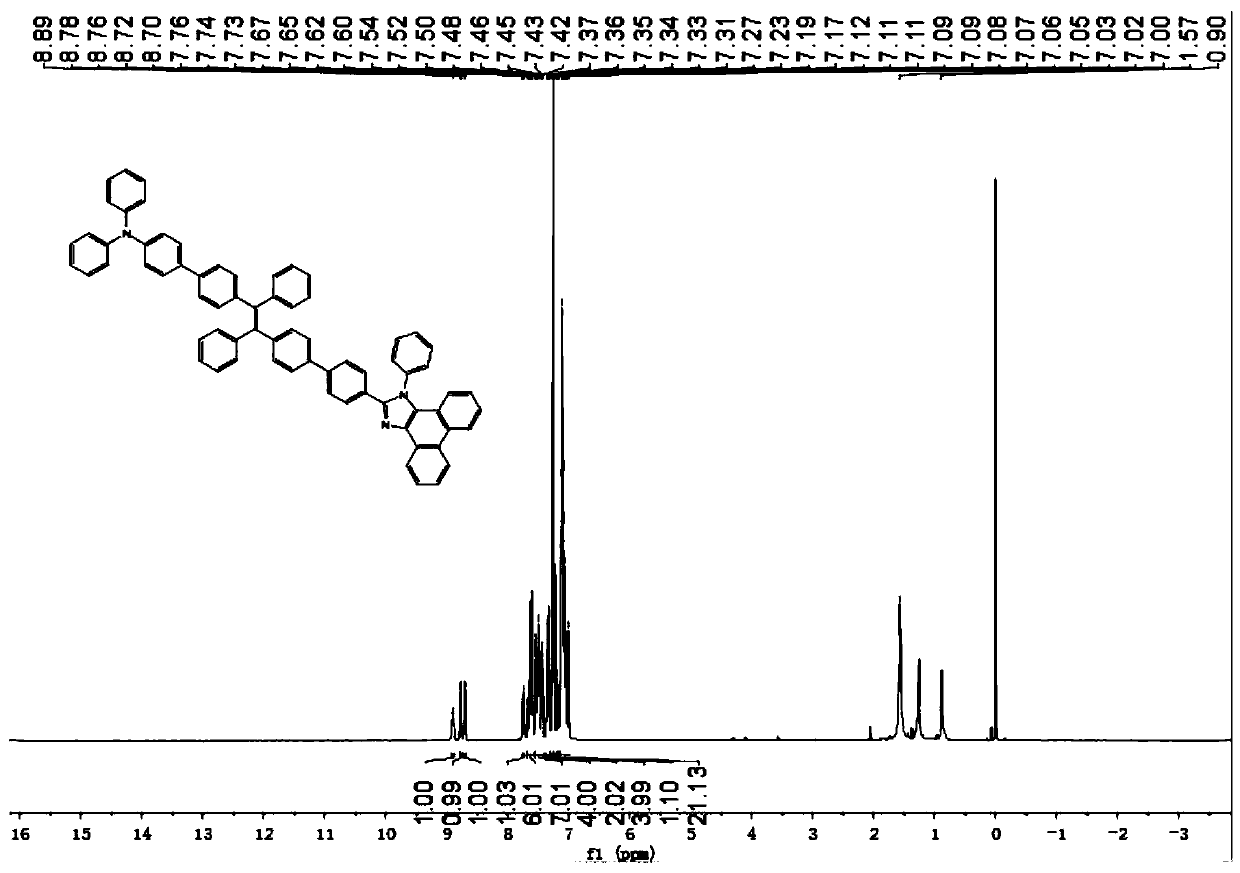
[0069] The preparation of the phenanthroimidazole of embodiment 2 triphenylamine substitution-tetrastyryl modification
[0070] The preparation process of the phenanthrene imidazole substituted by triphenylamine-tetrastyryl modification, steps 1 to 3 are the same as in Example 1, and steps 4 and 5 are as follows:
[0071] Step 4: Preparation of 4-bromotriphenylamine tetraphenylethylene
[0072] 4-triphenylamine benzophenone (1.53g, 3.60mmol) and 4-bromobenzophenone (0.80g, 7.20mmol) zinc powder (2.0g) were added in a 100ml two-necked flask, and the flask was placed under vacuum Evacuate and replace in dry nitrogen three times, then add 50ml of THF to remove water and oxygen, and then stir in a low-temperature reactor at -10°C (30 minutes). Then add 6ml of TiCl dropwise with a needle 4 , and stirred at -10°C for 30 minutes. After the reaction was warmed to room temperature, the reaction was quenched with 1M hydrochloric acid, and extracted with saturated brine and dichloromet...
Embodiment 3
[0081] Embodiment 3 performance test
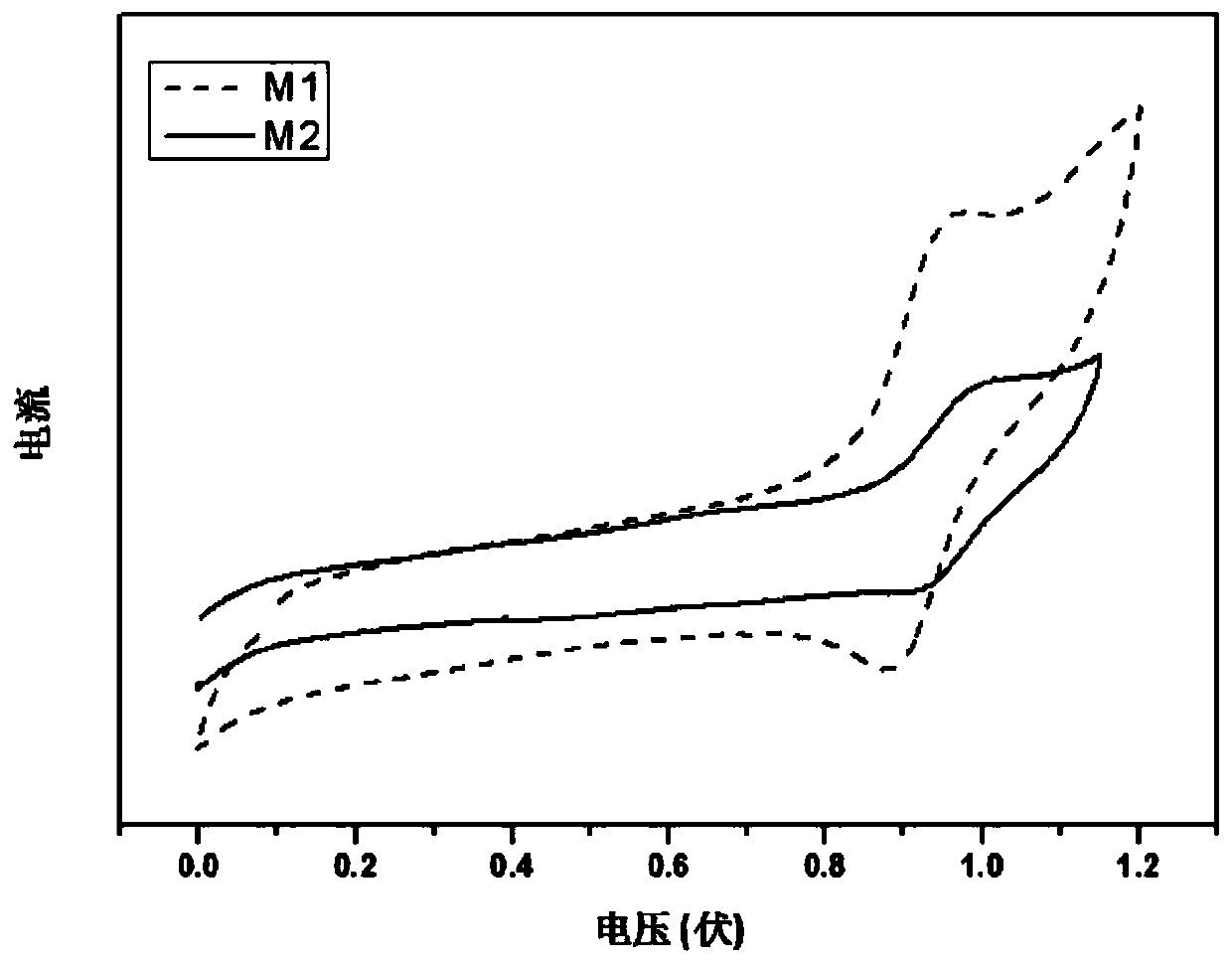
[0082] Taking the compounds M1 and M2 prepared in Example 1 and Example 2 as test objects, their photophysical properties and other luminescent properties were tested, and the test results were as follows: Figure 3 to Figure 8 shown.
[0083] image 3 The oxidation-reduction potentials of molecules M1 and M2 were measured by cyclic voltammetry on a Metrohm PGSTAT 302N high-precision electrochemical workstation. Such as image 3 As shown, wherein M1 is triphenylamine-substituted-triphenylethylene-modified phenanthroimidazole, and M2 is triphenylamine-substituted-tetraphenylethylene-modified phenanthroimidazole. The oxidation potentials of both are relatively high, indicating that the highest occupied orbital is relatively high, which is conducive to the injection and transmission of holes and electrons, and can be used for vacuum evaporation to make organic electroluminescent devices.
[0084] Figure 4 The fluorescence emission spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com