Preparation method of ferric phosphate with high tap density
A technology of tap density and iron phosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of high nucleation rate, low utilization rate of phosphorus resources, and excessive excess, so as to improve the growth of crystal nuclei rate, avoiding environmental protection treatment costs, and inhibiting the effect of nucleation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
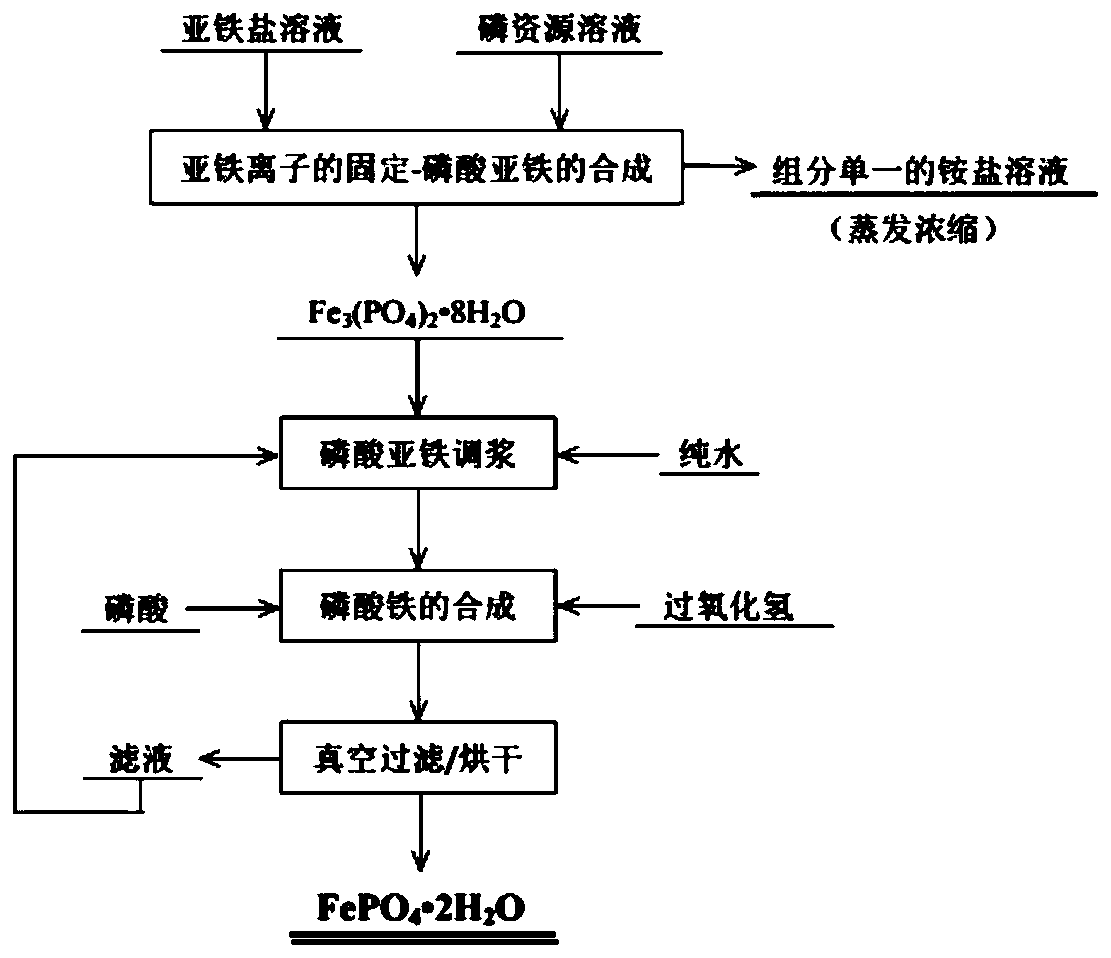
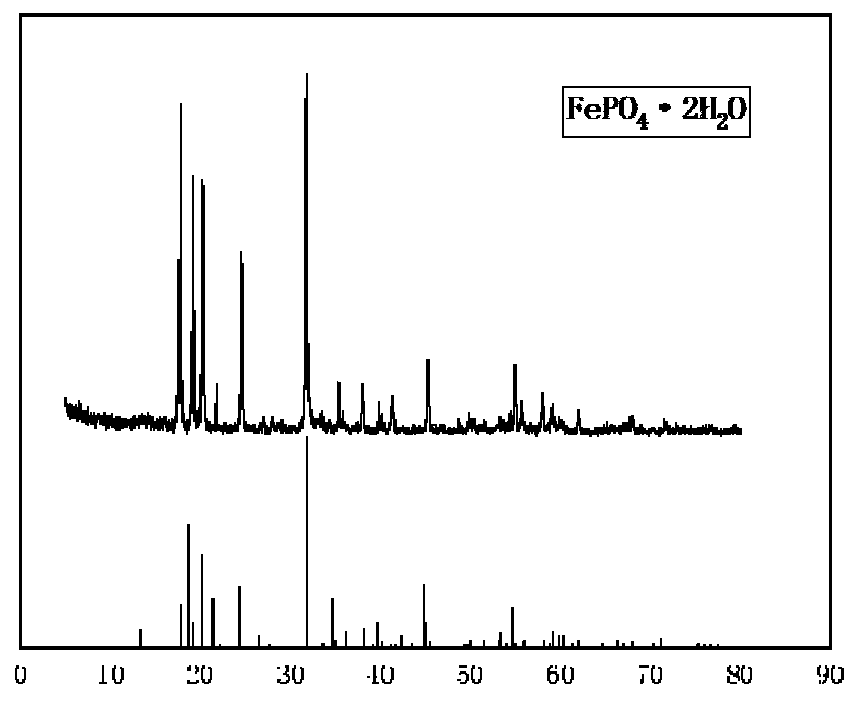
[0043] (1) Synthesis of ferrous phosphate
[0044] Weigh 25.02g of ferrous sulfate heptahydrate and dissolve it in 100ml of boiled deionized water to form a 0.9mol / L ferrous sulfate solution; weigh 7.92g of diammonium hydrogen phosphate and dissolve it in 100ml of deionized water to form a For the diammonium hydrogen phosphate solution of L, adjust the pH of diammonium hydrogen phosphate to 9.68 with ammonia water (1+1). Add the diammonium hydrogen phosphate solution into the reaction kettle equipped with the ferrous sulfate solution with a peristaltic pump at a rate of 20ml / min. After adding the diammonium hydrogen phosphate, the pH of the mixed solution is 4.67. Heated in a water bath to 30°C for 1.0h.
[0045] (2) Preparation of ferrous phosphate suspension
[0046] Wash the precipitate obtained by the reaction in step (1) with suction filtration and water, weigh 42.36 g of ferrous phosphate after washing, add ferrous phosphate to the reaction kettle, add 423 ml of deioni...
Embodiment 2
[0052] (1) Synthesis of ferrous phosphate
[0053] Weigh 33.36g of ferrous sulfate heptahydrate and dissolve it in 100ml of boiled deionized water to form a 1.2mol / L ferrous sulfate solution; weigh 10.56g of diammonium hydrogen phosphate and dissolve it in 100ml of deionized water to form a For the diammonium hydrogen phosphate solution of L, adjust the pH of diammonium hydrogen phosphate to 9.7 with ammonia water (1+1). Add the diammonium hydrogen phosphate solution into the reaction kettle equipped with the ferrous sulfate solution with a peristaltic pump at a rate of 20ml / min. After adding the diammonium hydrogen phosphate, the pH of the mixed solution is 4.7. Heated in a water bath to 30°C for 1.0h.
[0054] (2) Preparation of ferrous phosphate suspension
[0055] Wash the precipitate obtained by the reaction in step (1) by suction filtration and water, weigh 44g of ferrous phosphate after washing, add ferrous phosphate into the reaction kettle, add 440ml of deionized wa...
Embodiment 3
[0061] (1) Synthesis of ferrous phosphate
[0062] Weigh 33.36g of ferrous sulfate heptahydrate and dissolve it in 100ml of boiled deionized water to form a 1.2mol / L ferrous sulfate solution; weigh 10.56g of diammonium hydrogen phosphate and dissolve it in 100ml of deionized water to form a For the diammonium hydrogen phosphate solution of L, adjust the pH of diammonium hydrogen phosphate to 9.72 with ammonia water (1+1). Add the diammonium hydrogen phosphate solution into the reaction kettle equipped with the ferrous sulfate solution with a peristaltic pump at a rate of 20ml / min. After adding the diammonium hydrogen phosphate, the pH of the mixed solution is 4.7. Heated in a water bath to 30°C for 1.0h.
[0063] (2) Preparation of ferrous phosphate suspension
[0064] Wash the precipitate obtained by the reaction in step (1) by suction filtration and water, weigh the mass of ferrous phosphate after washing to 42.36g, add ferrous phosphate into the reaction kettle, add 847ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com