Preparation method of spiro indolone compound in water phase
A technology of spiro indolinone and compound, which is applied in the field of catalytic synthesis of fine chemical industry, can solve the problems such as the limitation of yield and substrate universality, and achieve the effects of easy reaction scale, short process flow and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063]
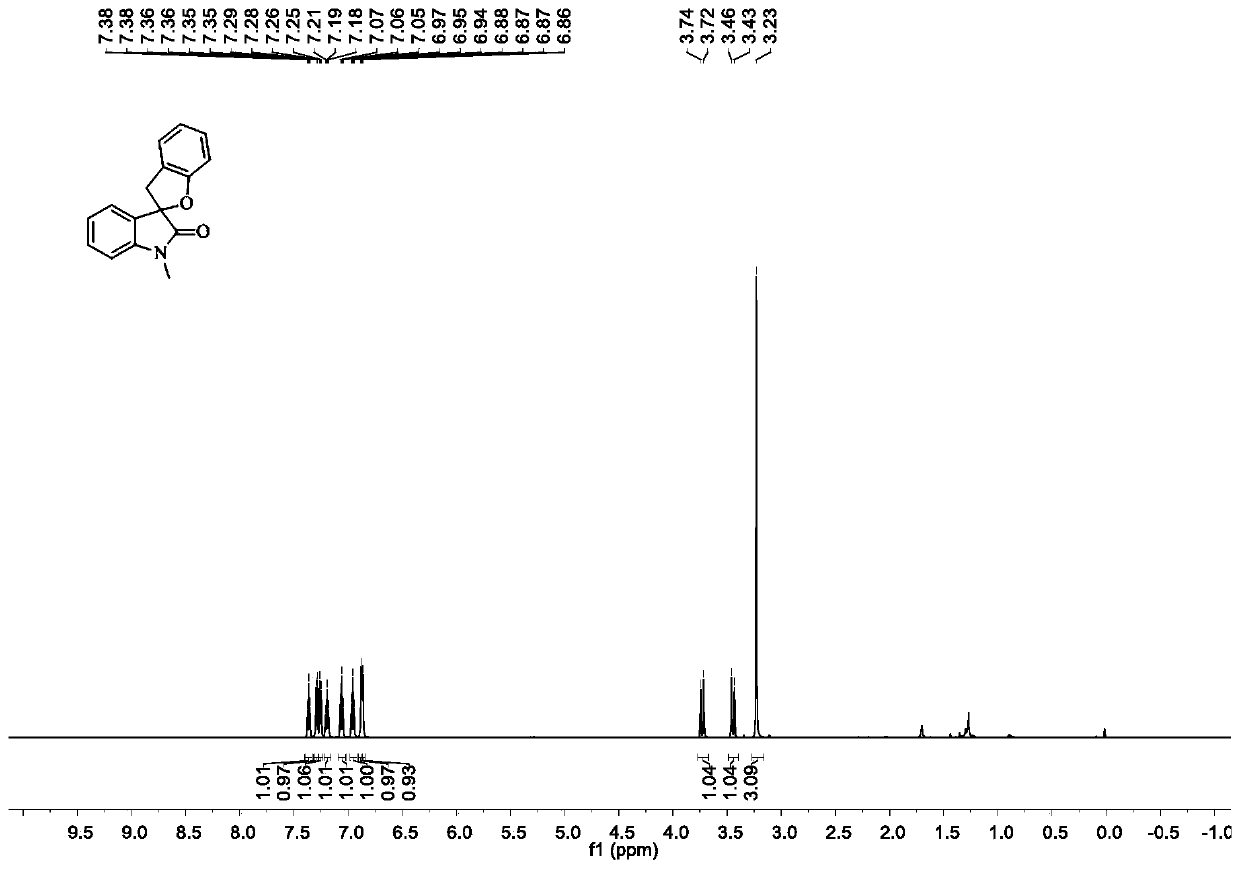
[0064] Add substrate (0.2mmol, 50.6mg), trimethyldodecyl ammonium iodide (0.02mmol, 7.2mg), 2ml of water, and finally hydrogen peroxide (0.6mmol, 60 μL). The reaction mixture was stirred at room temperature for 24 h, and the reaction was stopped. Ethyl acetate (5 mL*4) was added to the reaction system for extraction. The combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to obtain a crude product. The obtained crude product was purified and separated by column chromatography to obtain a white solid product with an isolated yield of 98%. figure 1 It is the proton nuclear magnetic resonance spectrum of the target product obtained in Example 1.
[0065] 1 H NMR (600MHz, CDCl 3 )δ7.36(td, J=7.8,1.0Hz,1H),7.29(d,J=7.3Hz,1H),7.25(d,J=7.5Hz,1H),7.19(t,J=7.7Hz, 1H), 7.06(t, J=7.5Hz, 1H), 6.95(t, J=7.4Hz, 1H), 6.88(d, J=4.3Hz, 1H), 6.86(d, J=4.2Hz, 1H) , 3.73 (d, J = 15.8Hz, 1H), 3.45 (d, J = 15.8Hz, 1H), 3.23 (s, 3H)....
Embodiment 2
[0067]
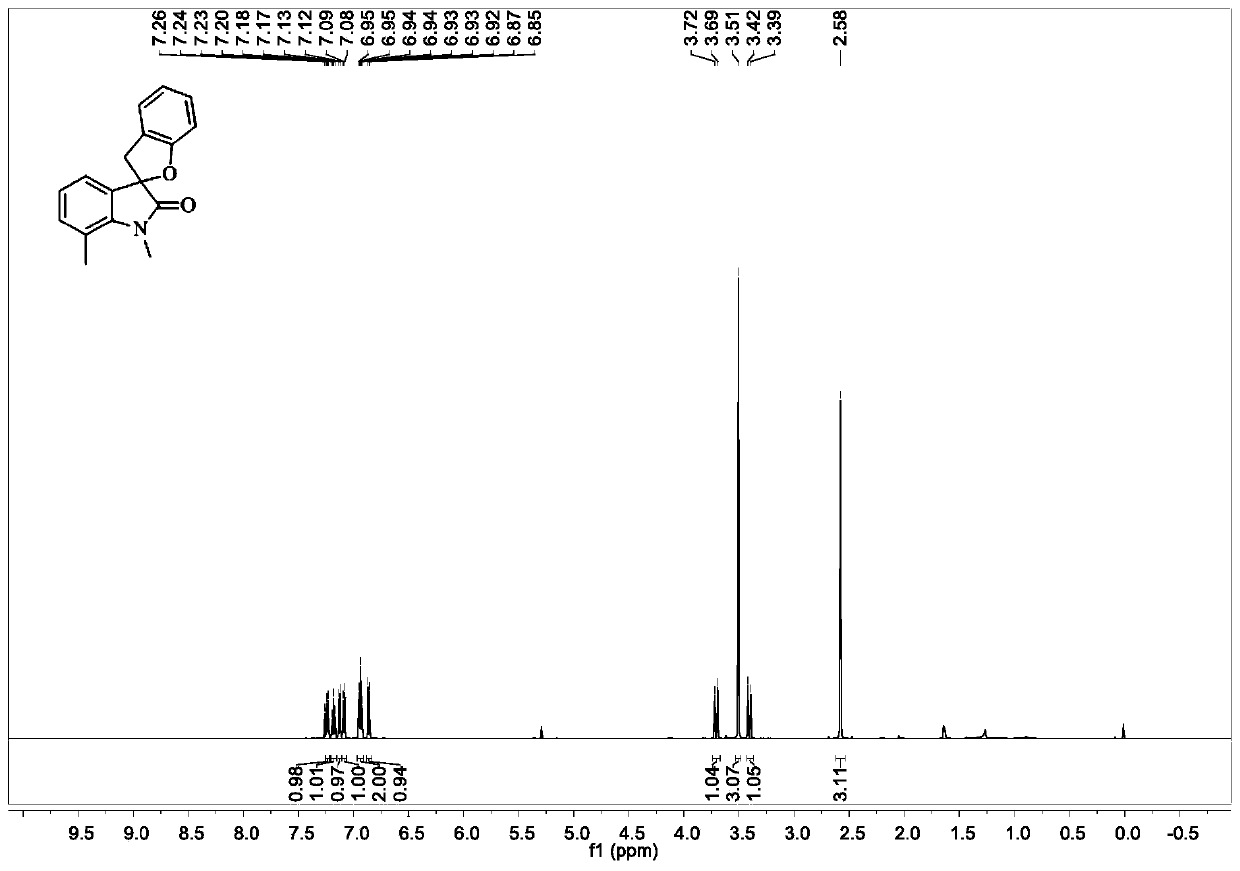
[0068] Add substrate (0.2mmol, 53.4mg), trimethyldodecyl ammonium iodide (0.02mmol, 7.2mg), 2ml of water, and finally hydrogen peroxide (0.6mmol, 60 μL). The reaction mixture was stirred at room temperature for 24 h, and the reaction was stopped. Ethyl acetate (5 mL*4) was added to the reaction system for extraction. The combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to obtain a crude product. The obtained crude product was purified and separated by column chromatography to obtain a white solid product with an isolated yield of 88%. figure 2 It is the proton nuclear magnetic resonance spectrum of the target product obtained in Example 2.
[0069] 1 H NMR (600MHz, CDCl 3 )δ7.24(d, J=7.2Hz, 1H), 7.18(t, J=7.6Hz, 1H), 7.13(d, J=7.3Hz, 1H), 7.09(d, J=7.7Hz, 1H) ,6.96–6.91(m,2H),6.86(d,J=8.0Hz,1H),3.71(d,J=15.8Hz,1H),3.51(s,3H),3.41(d,J=15.8Hz, 1H), 2.58(s, 3H).
Embodiment 3
[0071]
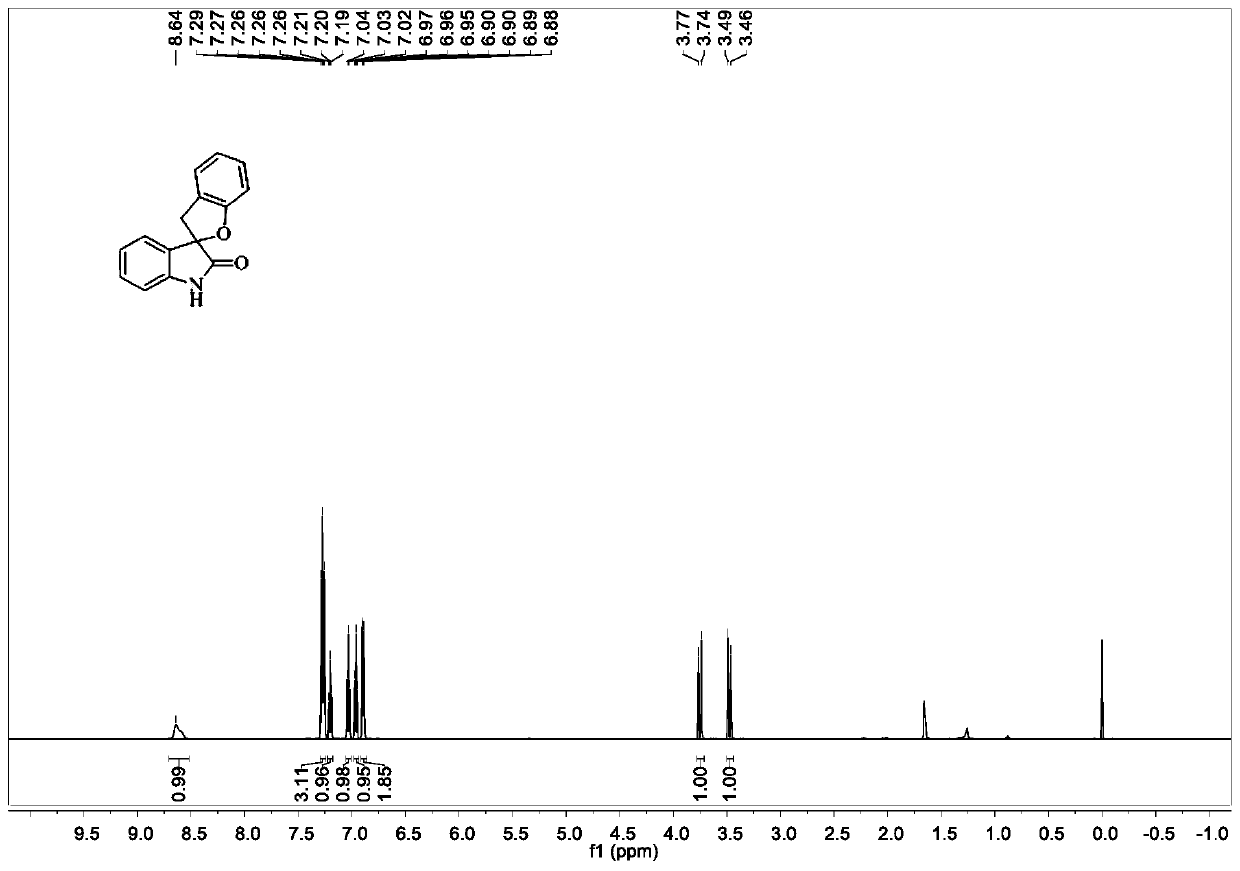
[0072] Add substrate (0.2mmol, 47.8mg), trimethyldodecyl ammonium iodide (0.02mmol, 7.2mg), 2ml of water, and finally hydrogen peroxide (0.6mmol, 60 μL). The reaction mixture was stirred at room temperature for 24 h, and the reaction was stopped. Ethyl acetate (5 mL*4) was added to the reaction system for extraction. The combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to obtain a crude product. The obtained crude product was purified and separated by column chromatography to obtain a white solid product with an isolated yield of 87%. image 3 It is the proton nuclear magnetic resonance spectrum of the target product obtained in Example 3.
[0073] 1 H NMR (600MHz, CDCl 3 )δ8.64(s,1H),7.30–7.24(m,3H),7.20(t,J=7.6Hz,1H),7.03(t,J=7.5Hz,1H),6.96(t,J=7.4 Hz, 1H), 6.89 (dd, J=8.0, 2.2Hz, 2H), 3.75 (d, J=15.9Hz, 1H), 3.48 (d, J=15.9Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com