Red phosphor activated by europium ions Eu3+, preparation method and application
A red phosphor, europium ion technology, applied in chemical instruments and methods, luminescent materials, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
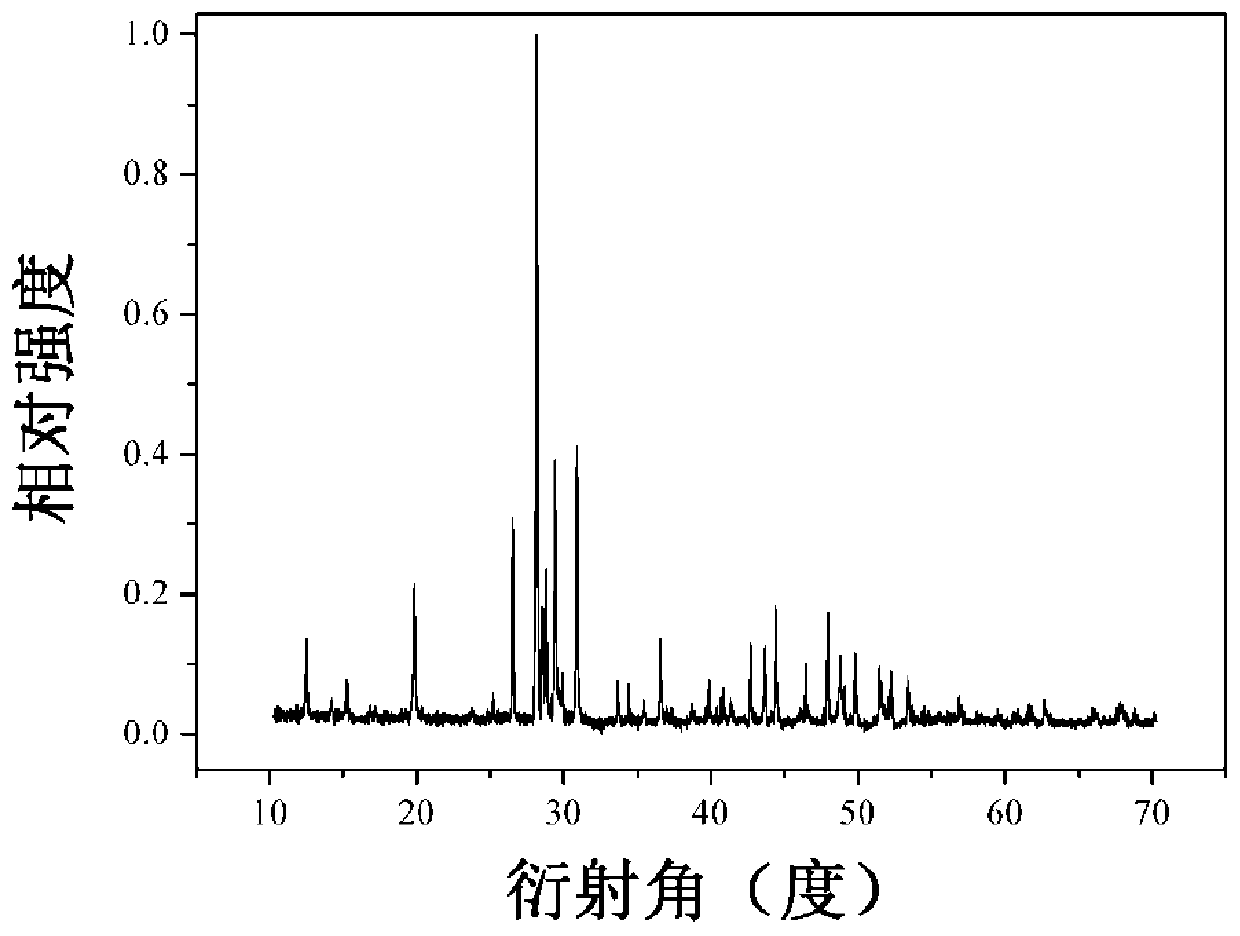
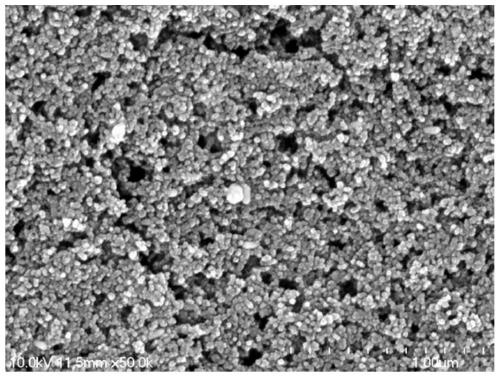
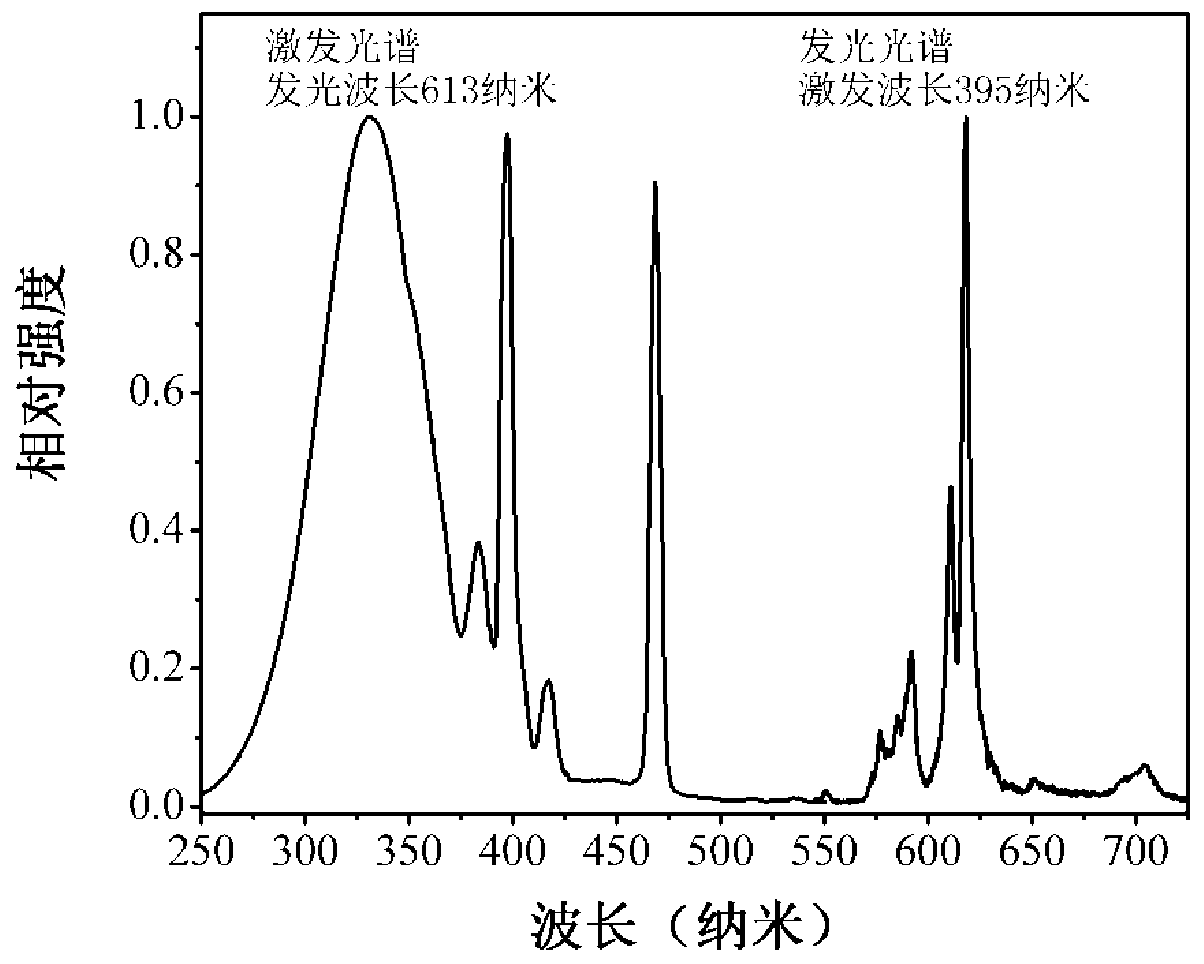
Image
Examples
Embodiment 1
[0032] Preparation of CaBiLa 0.88 Eu 0.12 V 2 o 9
[0033] According to the chemical formula CaBiLa 0.88 Eu 0.12 V 2 o 9 The stoichiometric ratio of the elements Ca, Bi, La, Eu and V in the medium, respectively weighed calcium nitrate Ca(NO 3 ) 2 : 1.18 g, bismuth nitrate Bi (NO 3 ) 3 ·5H 2 O: 2.436 grams, lanthanum nitrate La (NO 3 ) 3 ·6H 2 O: 2.574 grams, europium nitrate Eu (NO 3 ) 3 ·6H 2 O: 0.268 g, ammonium vanadate NH 4 VO 3 : 1.17g. The above reagents were dissolved in dilute nitric acid solution. Then add oxalic acid: 0.9 g to the calcium nitrate solution, add 0.9 g oxalic acid to the bismuth nitrate solution, add 0.792 g oxalic acid to the lanthanum nitrate solution, add 0.11 g oxalic acid to the europium nitrate solution, add 0.11 g to the ammonium vanadate solution Added oxalic acid: 1.8 grams. Each solution was continuously stirred until clear and transparent.
[0034] Mix and stir the above solutions, place the obtained mixed sol in an ove...
Embodiment 2
[0040] Preparation of CaBiLa 0.999 Eu 0.001 V 2 o 9
[0041] According to the chemical formula CaBiLa 0.999 Eu 0.001 V 2 o 9 The stoichiometric ratio of the elements Ca, Bi, La, Eu and V in the medium, weigh calcium carbonate CaCO 3 : 0.9 g, bismuth nitrate Bi(NO 3 ) 3 ·5H 2 O: 4.366 g, lanthanum oxide La 2 o 3 : 1.45 g, europium nitrate Eu (NO 3 ) 3 ·6H 2 O: 0.01 g, vanadium pentoxide V 2 o 5 : 1.638 g. Dissolve the above reagents in dilute nitric acid solution respectively, then add oxalic acid: 1.215 g to the calcium carbonate solution, add 1.215 g oxalic acid to the bismuth nitrate solution, add 0.6 g oxalic acid to the lanthanum oxide solution, and add 0.6 g to the europium nitrate solution Add oxalic acid: 0.01 g, add oxalic acid: 1.215 g in the vanadium pentoxide solution. Each solution was continuously stirred for a period of time until clear and transparent.
[0042] Mix and stir the above solutions, place the obtained mixed sol in an oven, set the...
Embodiment 3
[0045] Preparation of CaBiLa 0.85 Eu 0.15 V 2 o 9
[0046] According to the chemical formula CaBiLa 0.85 Eu 0.15 V 2 o 9 The stoichiometric ratio of the elements Ca, Bi, La, Eu and V in the medium, respectively weigh calcium oxide CaO: 0.504 g, bismuth carbonate (BiO) 2 CO 3 0.5H 2 O: 2.335 grams, lanthanum oxide La 2 o 3 : 1.464 g, europium oxide Eu 2 o 3 : 0.237 g, ammonium vanadate NH 4 VO 3 : 2.106 g. Dissolve the above reagents in dilute nitric acid solution respectively, then add oxalic acid: 1.215 grams in calcium oxide solution, add oxalic acid: 0.61 grams in bismuth carbonate solution, add oxalic acid: 0.61 grams in lanthanum oxide solution, add oxalic acid: 0.61 grams in europium oxide solution Add oxalic acid: 0.1 gram in the ammonium vanadate solution and add oxalic acid: 2.43 grams. Each solution is continuously stirred for a period of time until it is clear and transparent.
[0047] Mix and stir the above solutions, place the obtained mixed sol i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com