Preparation method of MoP-Zn3In2S6 composite nano material
A composite nanomaterial, mop-zn3in2s6 technology, which is applied in the field of nanomaterial preparation and photocatalysis, can solve the problems of thermodynamic difficulty in photo-splitting water for hydrogen production, low photo-generated electron-hole separation efficiency of photocatalyst, etc., and achieves improved photocatalytic performance, The effect of high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The present embodiment prepares ZIS6 nano material according to the following steps:
[0043] Step 1, weigh 2mmolInCl 3 4H 2 O, 3 mmol ZnSO 4 ·7H 2 O, 0.65g of cetyltrimethylammonium bromide (CTAB) and 12 mmol of thioacetamide (TAA) in a 100mL polytetrafluoroethylene cup, add 70mL of deionized water as a solvent, stir and dissolve to form a mixed solution;
[0044] Step 2, put the polytetrafluoroethylene cup in step 1 into the steel-jacketed kettle to seal and keep it at 160°C for 12 hours. After the reaction is completed, cool it down to room temperature naturally, and wash the obtained precipitate alternately with deionized water and absolute ethanol Several times, the final product was vacuum-dried at 60 °C for 10 h in ZIS6.
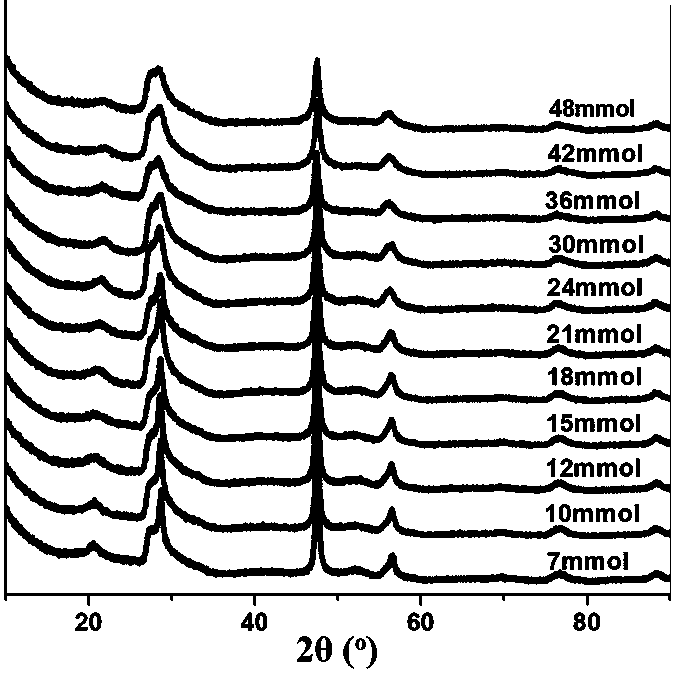
[0045] Such as figure 1 As shown, the XRD spectrum of ZIS6 prepared in this example is consistent with the hexagonal phase of pure ZIS6 (a = b = 3.85 Å, c = 21.79 Å, JCPDS No. 24-1453). The peaks with 2θ values of 20.3, 27.6, 28.8, 47.1...
Embodiment 2
[0049] The sulfur source used in step 1 was adjusted to L-cysteine, and the other steps were the same as in Example 1.
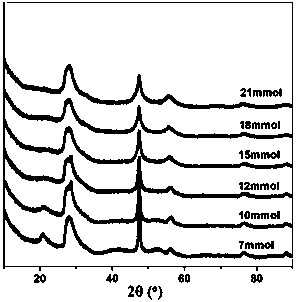
[0050] Such as figure 2 As shown, the ZIS6 synthesized by adding 12 mmol of L-cysteine is also a pure ZIS6 hexagonal phase.
[0051] Compared to ZIS6 synthesized by adding 12 mmol of TAA, L-cysteine decreased the (102) / (100) facet ratio.
[0052] It can be seen that the adjustment of the (102) and (100) crystal planes of ZIS6 can also be achieved by adjusting the sulfur source.
[0053] Change the amount of L-cysteine added, as the amount of L-cysteine increases from 7 to 21mmol, the obtained samples are all pure hexagonal ZIS6, and with the increase of the amount of L-cysteine The (110) crystal plane diffraction peak of ZIS6 is weakened. It can be seen that the increase of the amount of L-cysteine is not conducive to the formation of ZIS6 (110) crystal face.
Embodiment 3
[0055] The sulfur source used in step 1 is adjusted to thiourea, and other steps are the same as in Example 1.
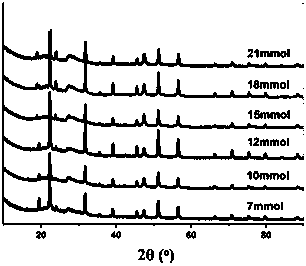
[0056] Such as image 3 As shown, when the sulfur source is thiourea, the addition amount from 7-21mmol can not get the pure phase of ZIS6, but the mixed phase of ZIS6 and ZnIn2S4.
[0057] The addition amount greater than 21mmol can not obtain ZIS6 with complete structure, what embodiment 1 and embodiment 2 obtain is pure ZIS6 hexagonal phase; And what this example obtains is mixed phase, find after activity comparison, the photocatalytic decomposition of formic acid of mixed phase produces Hydrogen activity is poor.
[0058] Among them, the peaks with 2θ values of 20.3, 27.6, 28.8, 47.1 and 56.5 degrees correspond to the (005), (100), (102), (110) and (200) crystal planes of ZIS6; the 2θ values are 22.4, 31.8, The peaks at 39.1, 45.7, 47.5, 51.3, 56.6 and 70.7 degrees correspond to the (006), (105), (108), (010), (112), (012), (203) and (017) crystals of ZnI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com