A kind of human fibroblast growth factor 18 and its soluble recombinant expression method, preparation method, preparation and application
A technology of human fibroblasts and growth factors, which is applied to the preparation methods of fibroblast growth factors and peptides, growth factors/inducing factors, etc., can solve the problems of complicated process, unstable structure, harsh renaturation conditions, etc., and achieve The process is simple, the structure is stable, and it is beneficial to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A preparation method of human fibroblast growth factor 18, comprising the steps of:
[0043] (1) low-temperature crushing by pressure homogenization, and centrifugation to collect the supernatant;
[0044] (2) Anion chromatography was used to capture FGF18ΔN in the supernatant of cell disruption;
[0045] (3) Using copper ions as the correct pairing accelerator for disulfide bonds;
[0046] (4) After correct pairing of disulfide bonds, heparin affinity chromatography captures the target product.
[0047] The preparation method of human fibroblast growth factor 18 disclosed in the present invention comprises the following specific steps:
[0048] S1 selects the expression system containing the T7 promoter as the expression plasmid vector, and the corresponding Escherichia coli as the host strain;
[0049] S2 selects two suitable endonucleases, and then double-digests the plasmid vector and the N-terminal deletion of the gene of human fibroblast growth factor 18, then ...
example 1
[0071] Example 1. Construction and inducible expression of genetically engineered strains with N-terminal deletion of human fibroblast growth factor 18
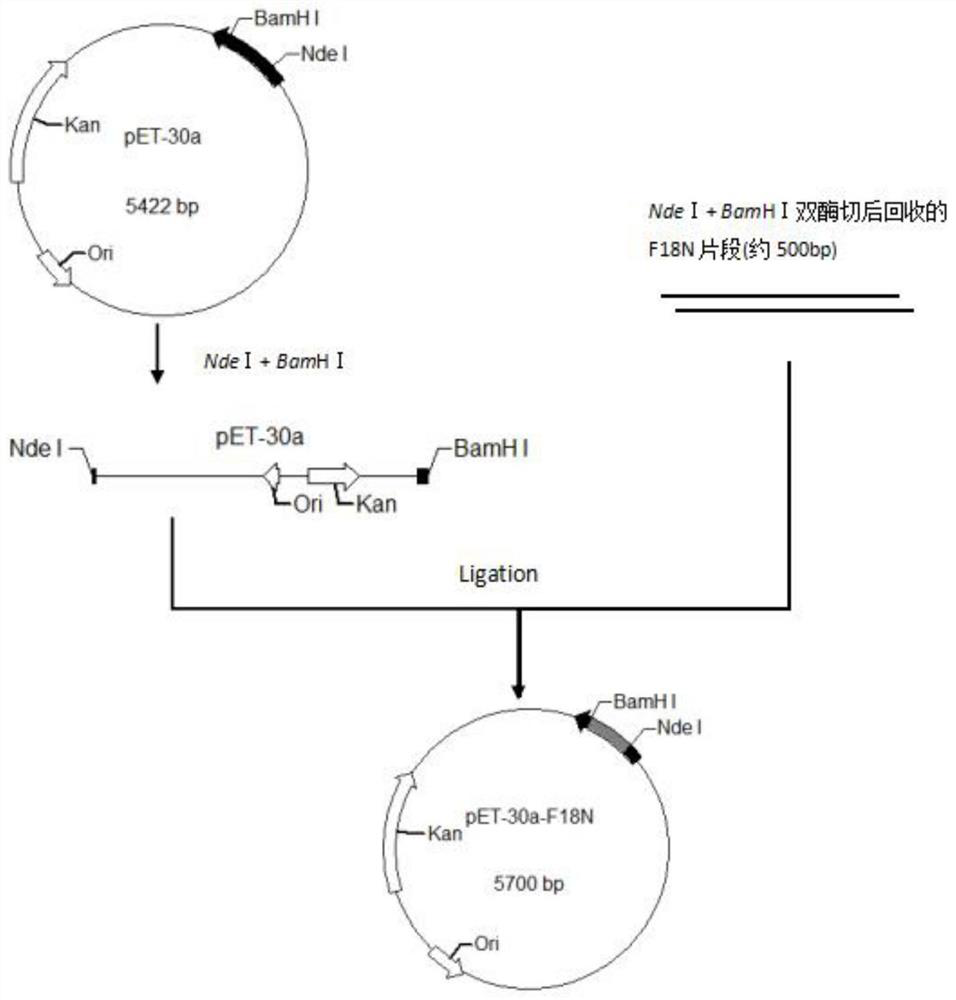
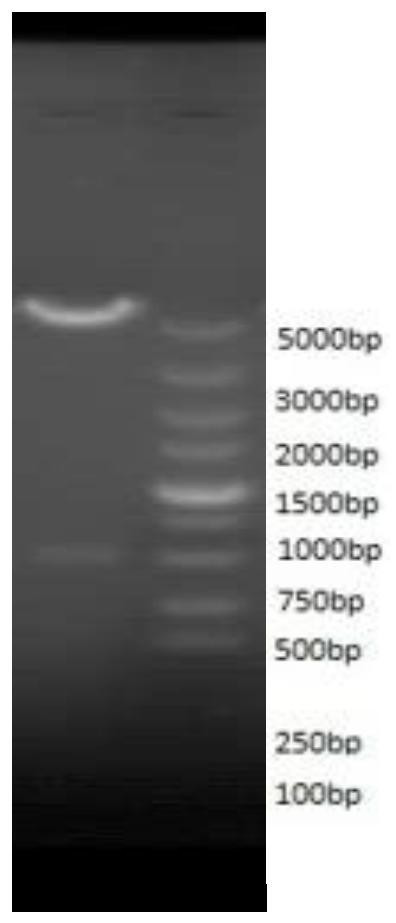
[0072] The cDNA sequence (SEQ ID NO:2) was designed based on the amino acid sequence of human fibroblast growth factor 18 (SEQ ID NO:1) deleted at the N-terminus. The whole gene was synthesized and inserted into the vector plasmid pUC-57 to obtain the pUC-57-FGF18ΔN plasmid. By using NdeI and BamHI restriction sites, the FGF18△N gene was connected to the vector plasmid pET-30a to construct the pET-30a-FGF△N recombinant plasmid, which was transformed into E. coli expression host strain BL21(DE3)PlysS , After expression screening, pET-30a-FGF18ΔN / BL21(DE3)PlysS recombinant engineering bacteria were constructed. The engineering strain is streaked and inoculated, and the bacterial lawn is selected and inoculated in a test tube containing a medium. After activation, it is transferred to the triangular agent and cultivated overnig...
example 2
[0073] Example 2. Disulfide bond formation with N-terminal deletion of human fibroblast growth factor 18
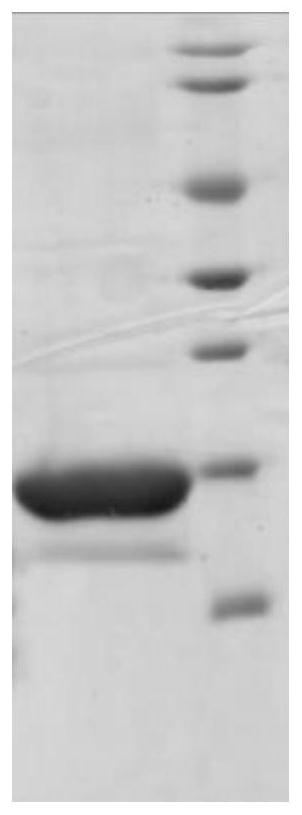
[0074] After the bacteria were resuspended, high-pressure homogenization was used to break the bacteria. FGF18ΔN was captured by anion chromatography, diluted with diluent (20mM Tris, pH 7.5) in an equal volume ratio of 2:1, and the final concentration of 5uM copper ions was added. HPLC detection showed that the pairing rate of disulfide bonds was not less than 90%, and FGF18ΔN with high activity was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com