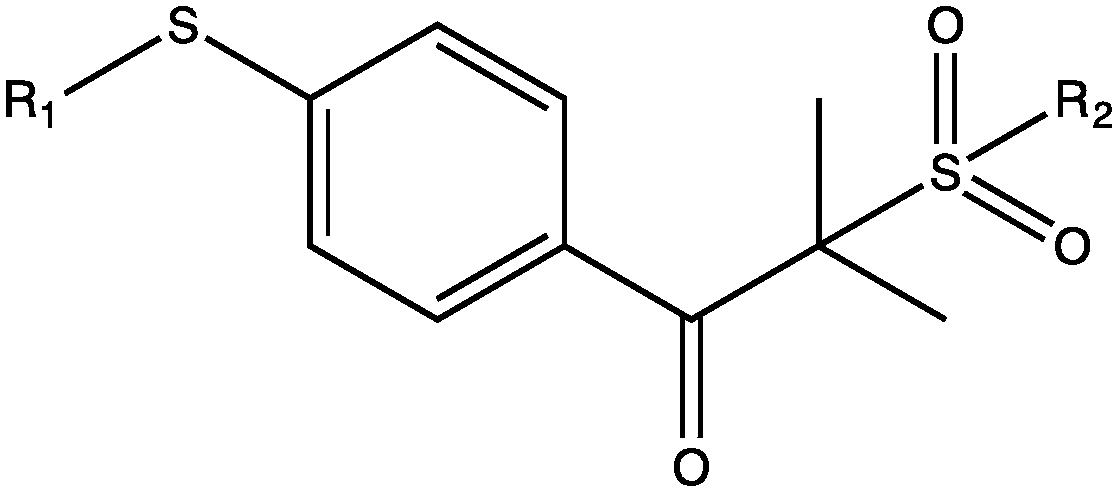
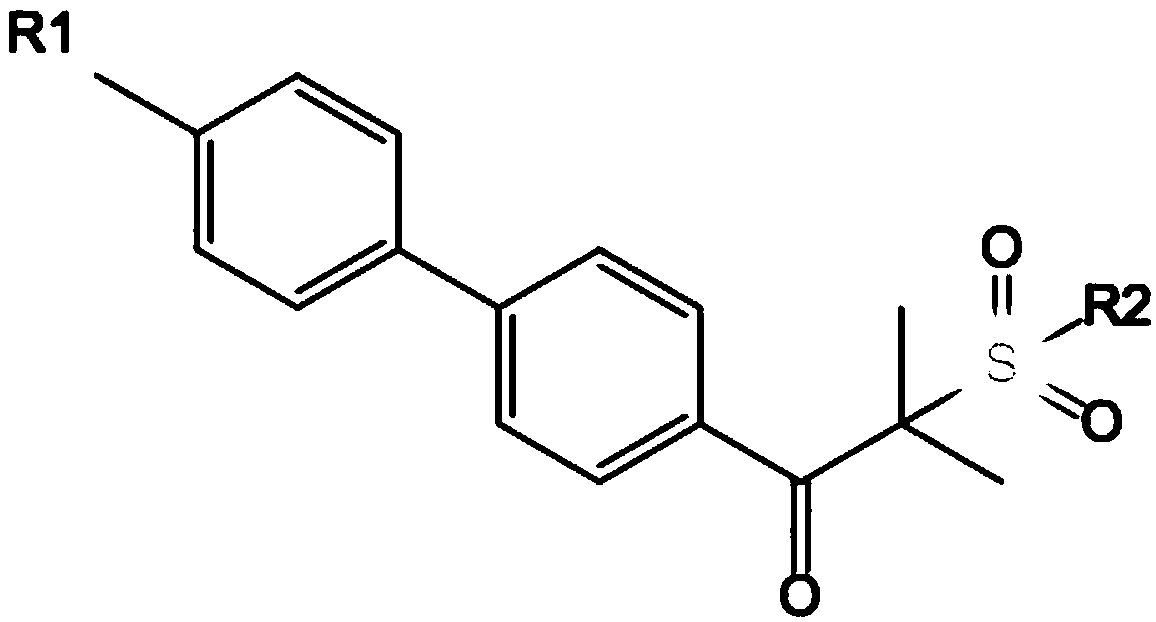
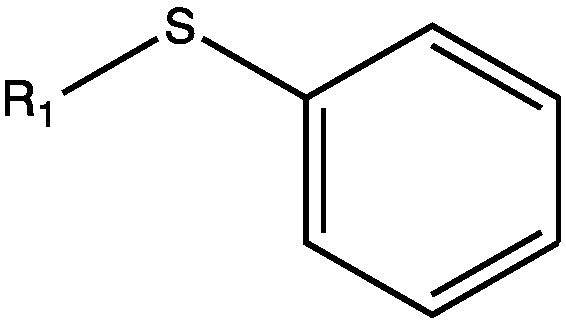
Novel alpha-aminoketone LED (light-emitting diode) initiator and a preparation method thereof
An aminoketone and initiator technology, which is applied in the field of new α-aminoketone LED initiators and their preparation, can solve the problems of poor initiation efficiency, easy sublimation of photoinitiator 907, and inability to meet the non-benzeneization of inks, and achieves satisfactory results. No benzene, high environmental efficiency, and the effect of solving volatilization and migration problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of a novel α-aminoketone LED initiator, comprising the following steps:
[0037] Step (1): Friedel-Crafts reaction of the raw material and acid halide in an organic solvent under the catalysis of aluminum trichloride or zinc chloride to generate intermediate a, wherein the raw material is the general formula (Ⅲ):
[0038]
[0039] Or general formula (Ⅳ):
[0040]
[0041] Step (2): The intermediate a generated in step (1) undergoes a halogenation reaction with a halogen in an organic solvent to obtain an intermediate b, wherein the intermediate b is the general formula (Ⅴ):
[0042]
[0043] Or general formula (Ⅵ):
[0044]
[0045] Step (3): The intermediate b generated in step (2) reacts with the dehalogenating agent represented by the general formula (VII) in a solvent to obtain an α-aminoketone LED initiator, wherein the general formula (VII) is:
[0046]
[0047] Wherein general formula (III), general formula (IV) and general f...
Embodiment 1
[0051] Preparation of 2-methyl-2-methylsulfonyl-1-(4-methylthiophenyl)acetone
[0052]
[0053] Put 12.4g of sulfide anisole, 16g of anhydrous aluminum trichloride and 50ml of dichloromethane into a 100mL four-necked flask under nitrogen protection, start stirring and cool down to 0-5°C, slowly add 10.6g of isobutyryl chloride dropwise and Keep the temperature below 5°C, continue to react for 2 hours after the dropwise addition, then hydrolyze to separate the organic layer, add 20g of bromine to react for 1 hour, separate the unreacted bromine, add 17.8g of sodium p-toluenesulfinate, After stirring for 2 hours, the organic phase was separated by filtration, the solvent was evaporated and recrystallized with methanol to obtain 29.6 g of product with a purity of 98.6% and a yield of 85%.
Embodiment 2
[0055] Preparation of 2-methyl-2-methylsulfonyl-1-(4'-acetyldiphenylsulfide)acetone
[0056]
[0057] Put 22.8g of 4'-acetyl diphenyl sulfide, 16g of anhydrous aluminum trichloride and 50ml of dichloromethane into a 100mL four-necked flask under nitrogen protection, start stirring and cool down to 0-5°C, slowly add 10.6 g isobutyryl chloride and keep the temperature below 5°C, continue to react for 2 hours after the dropwise addition, then hydrolyze and separate the organic layer, add 20g of bromine and react for 1 hour, separate the unreacted bromine, add 17.8g of p-toluene Sodium sulfinate was stirred for 2 hours, and the organic phase was separated by filtration. The solvent was evaporated and recrystallized with methanol to obtain 37.1 g of product with a purity of 98.8% and a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com