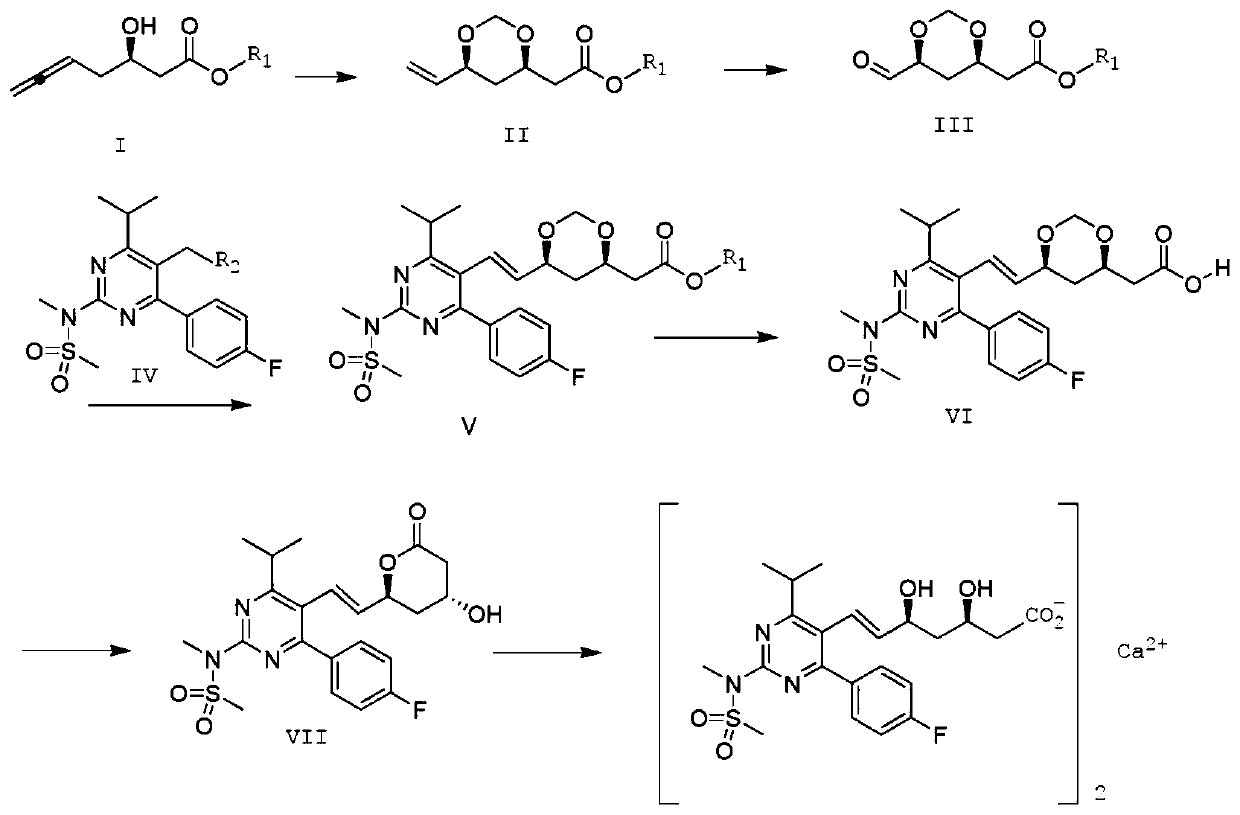
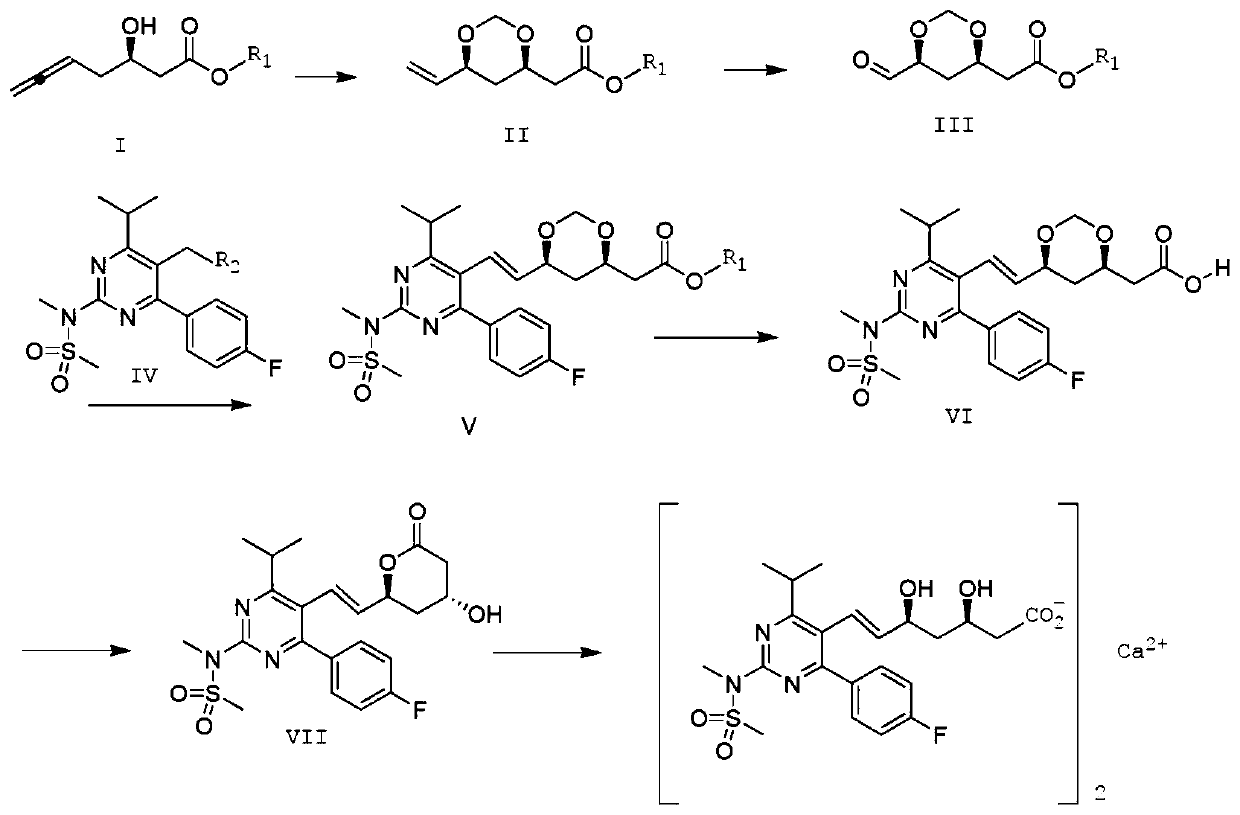
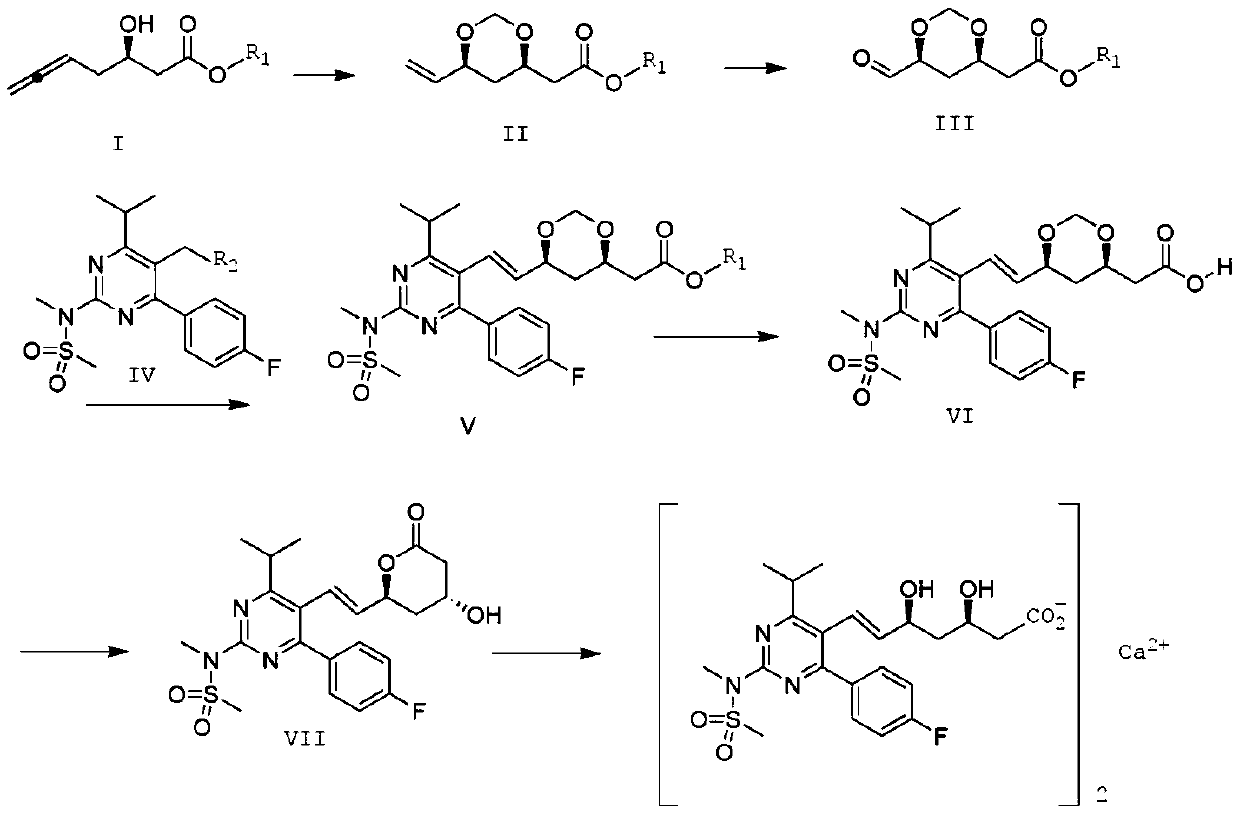
Preparation method of rosuvastatin calcium
A technology of rosuvastatin calcium and its compounds, which is applied in the field of drug synthesis, can solve problems such as unfavorable industrial production, and achieve the effects of realizing industrial production, increasing synthesis yield, and avoiding harsh conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049]Compound II was prepared according to the method of Bernhard Breit et al. (Org. Lett. 2018, 20, 3286-3290). Add Pd(PPh 3 ) 4 (2.67g, 2.3mmol, 2.0mol%), (S)-(-)-SEGphos (3.52g, 5.77mmol, 5.0mol%) and p-toluenesulfonic acid (1.98g, 11.54mmol, 10mol%), nitrogen After 5min, add formula I compound (18.0g, 115.4mmol, 1.0 equivalent), 37% aqueous formaldehyde solution (18.6mL, 248.4mmol) and freshly distilled toluene (300mL), heat up to 85°C, stir for 10 hours, and cool to room temperature Afterwards, the solvent was removed under reduced pressure, and by flash column chromatography (silica gel column, n-hexane:EtOA C =2:1), to obtain a colorless transparent liquid compound II (20.2g, 94%, d.r.=97:3). 1 H-NMR (400MHz, CDC1 3 ):δ=5.83(ddd,1H),5.26(ddd,1H),5.13(ddd,1H),5.09(d,1H),4.74(d,1H),4.13-4.07(m,2H),3.67( s,3H), 2.61(dd,1H), 2.43(dd,1H), 1.70(m,1H), 1.49(ddd,1H), basically similar to those reported in the literature.
[0050] According to the operating me...
Embodiment 2
[0054]
[0055] Dissolve 2.0g of the compound of formula II in 10ml of ethanol, add 0.2g of heteropolyacid H 3 PW 12 o 40 , dropwise added 30% H 2 o 2 10ml aqueous solution, the reaction temperature was controlled at 35°C, after 6 hours of reaction, the solvent was evaporated, water was added, extracted with ethyl acetate, and the organic phase was Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 2.0 g of the compound of formula III with a yield of 99%. 1 H-NMR (400MHz, CDC1 3 ):δ=9.56(d,1H),5.13(m,1H),4.76(d,1H),4.12-4.06(m,2H),3.68(s,3H),2.62(dd,1H),2.44( dd,1H), 1.72(m,1H), 1.50(ddd,1H).
[0056] According to the operation method of embodiment 2, the influence of the selection of oxidation system and catalyzer on this reaction has been studied, and the results are shown in table 2:
[0057] Table 2 The influence of different oxidation systems and reaction conditions on the reaction
[0058] oxidation system catal...
Embodiment 3
[0060]
[0061] Dissolve 6.78g (0.01mol) of the compound of formula IV and 1.88g (0.01mol) of the compound of formula III in 30ml of DMSO, stir to dissolve; raise the temperature to 80°C; add 4.14g (0.03mol) of potassium carbonate, stir, and keep warm at 80°C for 10h After the reaction, 80ml of water was added to precipitate a white solid, which was filtered, washed with saturated brine, washed with 5% ethanol, and dried to obtain 4.72g of white solid compound V with a yield of 93%, E:Z=97:3.
[0062] S(ESI)m / z:508.23(M+H) + ; 1 H-NMR (400MHz, CDC1 3 ):δ=7.62-7.60(m,2H),7.10-7.08(m,2H),6.59(dd,1H),5.44(dd,1H),5.11(d,1H),4.77(d,1H), 4.16-4.06(m,2H),3.69(s,3H),3.57(s,3H),3.50(s,3H),3.34(m,1H),2.63(dd,1H),2.44(dd,1H) ,1.60-1.57(m,1H),1.35(m,1H),1.26(d,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com