Synthesis, preparation and application of fluorescent probe for identifying Cys, GSH and HOCl on basis of molecular logic gate
A fluorescent probe and logic gate technology, applied in fluorescence/phosphorescence, luminescent materials, material excitation analysis, etc., can solve the problems of lack of high sensitivity and unclear exact changes, and achieve good selectivity, wide application value, and detection low limit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of fluorescent probe compound NPCC.
[0068] (1) Synthesis of Compound 1
[0069] The known compound 10-acetyl-9-hydroxy-2,3,6,7-tetrahydro-1H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-11 (5H)-ketone (897 mg, 3 mmol) and benzaldehyde (334 mg, 3.15 mmol) were dissolved in 25 mL of ethanol, 6–9 drops of piperidine were added during stirring, and refluxed for 14 hours. After the reaction was complete, cool to room temperature, filter with suction, wash the filter cake with ice-cold ethanol, and dry in vacuo to obtain compound 1 as a red solid (896.4 mg, yield 77.2%).
[0070] H NMR spectrum determination: 1 H NMR (400 MHz, CDCl 3 , ppm):1.92-1.99 (m, 4H), 2.76-2.85 (m, 4H), 3.29-3.32 (m, 4H),7.42 (m, 4H), 7.70 (m, 2H), 7.94 (d, 1 H, J =15.8 Hz), 8.46 (d, 1 H, J = 15.8 Hz).
[0071] Carbon NMR Spectrum Determination: 13 C NMR (100 MHz, CDCl 3 , ppm): δ = 20.14, 20.25,21.22, 27.48, 49.82, 50.25, 98.06, 103.11, 105.36, 118.70, 122.75, 124.07,128.88, 128.96, 13...
Embodiment 2
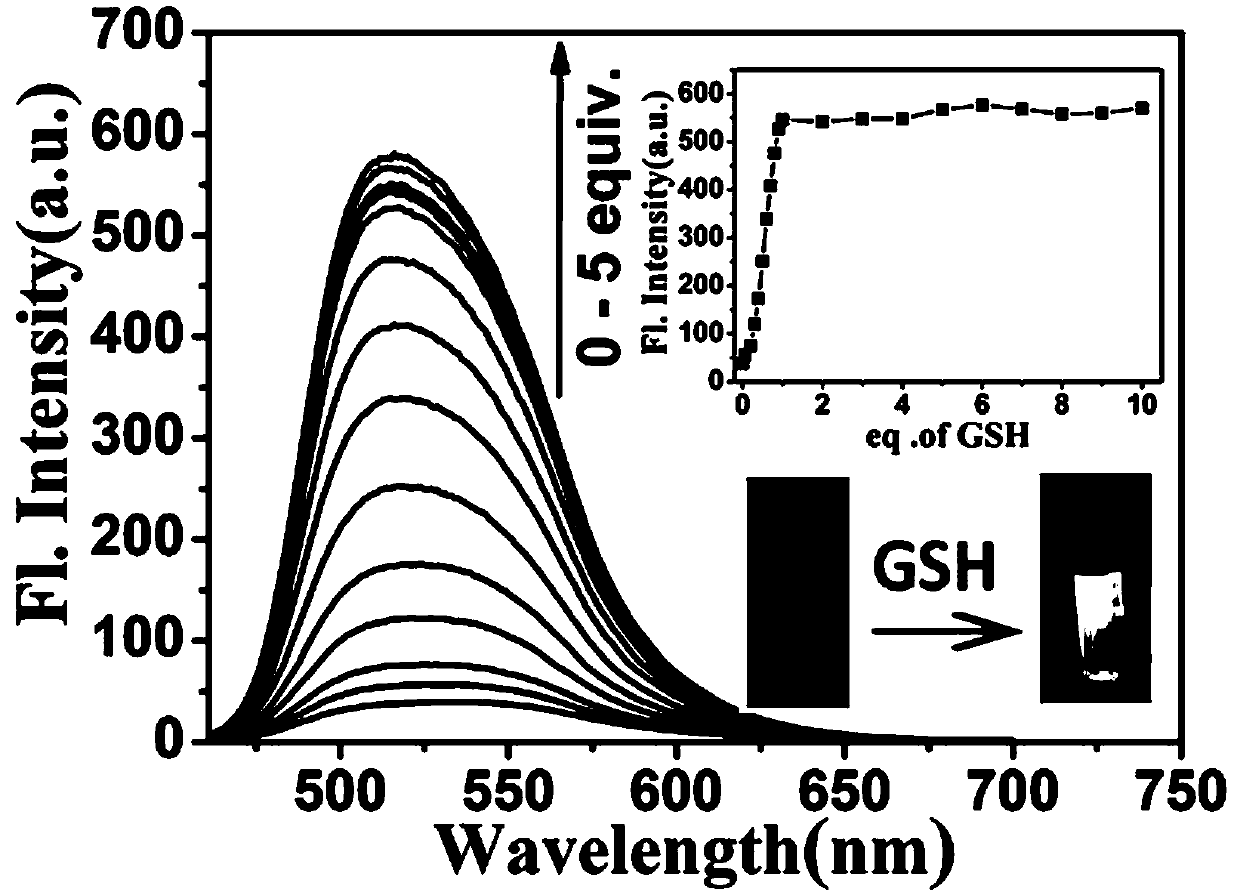
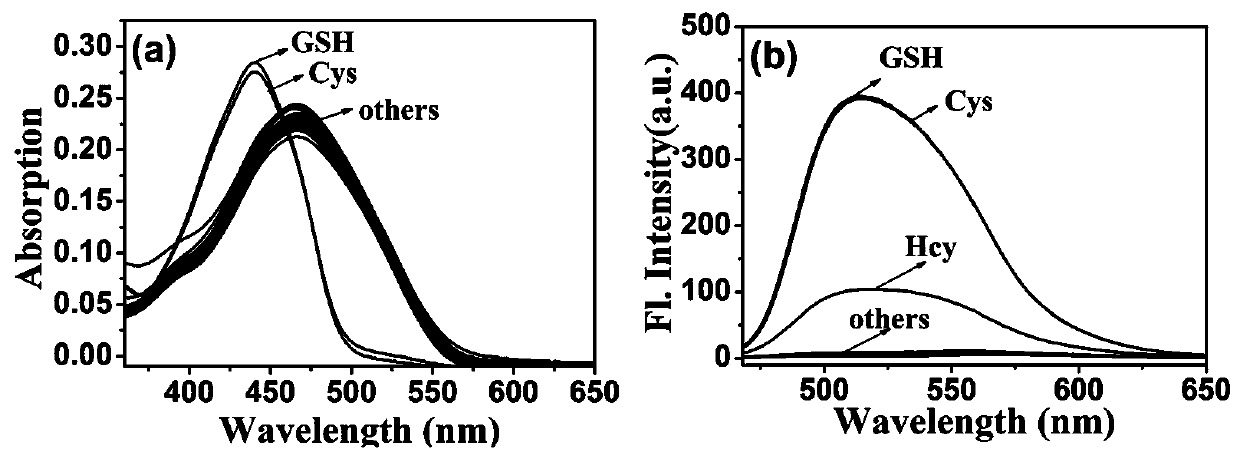
[0084] When the probe of the present invention interacts with Cys, GSH and HOCl, the ultraviolet-visible spectrum and the fluorescence emission spectrum change.
[0085] The probe prepared in Example 1 was dissolved in DMSO to prepare a probe master solution with a probe concentration of 1 mM. The test solution was Tris-HCl (10 mM, 1 mM CTAB) buffer solution, and the pH value of the buffer solution was 7.4. Take 30 μL of the probe mother solution and add it to 3 mL of the test solution to obtain the probe mixed solution. The final concentration of the probe in the mixed solution is 10 μmol / L. The UV-visible spectrum and fluorescence spectrum changes of the probes when they interacted with Cys, GSH and HOCl were tested with a UV-visible spectrophotometer and a fluorescence spectrophotometer. from figure 1 It can be seen that after adding Cys (10 μM) or GSH (10 μM), the maximum ultraviolet absorption peak of the probe blue-shifted from 471 nm to 441 nm, and the fluorescence in...
Embodiment 3
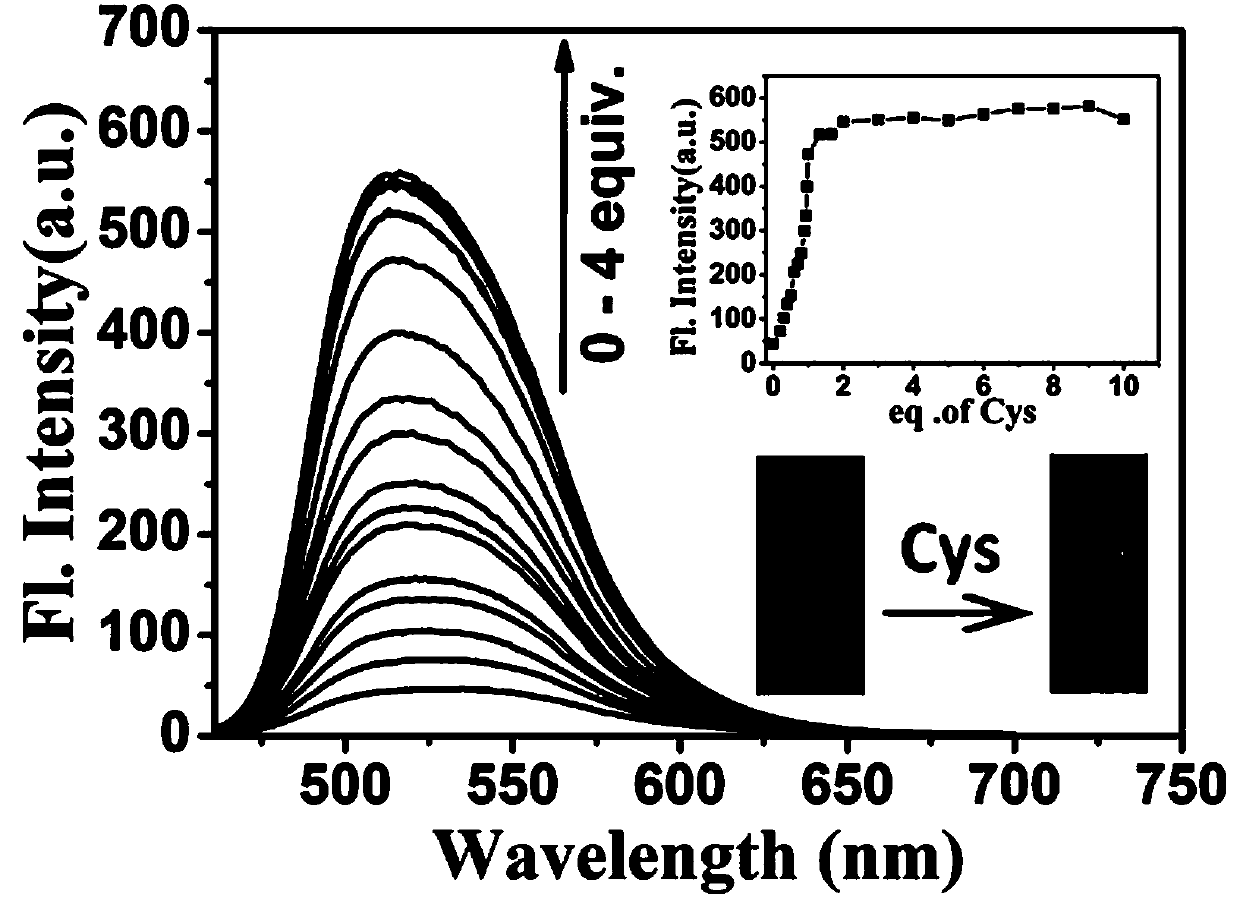
[0087] Fluorescence titration experiment of the probe of the present invention recognizing Cys.
[0088] Example 2 Preparation of the probe mother solution 30 μL was added to 3 mL of the test solution, the final concentration of the probe was 10 μmol / L, and different equivalents (0–4 equivalents) of the Cys mother solution were added, such as figure 2 As shown, with the increase of Cys concentration, the fluorescence intensity of the maximum fluorescence emission peak at 514 nm gradually increased, and the detection limit of the probe to recognize Cys was 8.95 nM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com