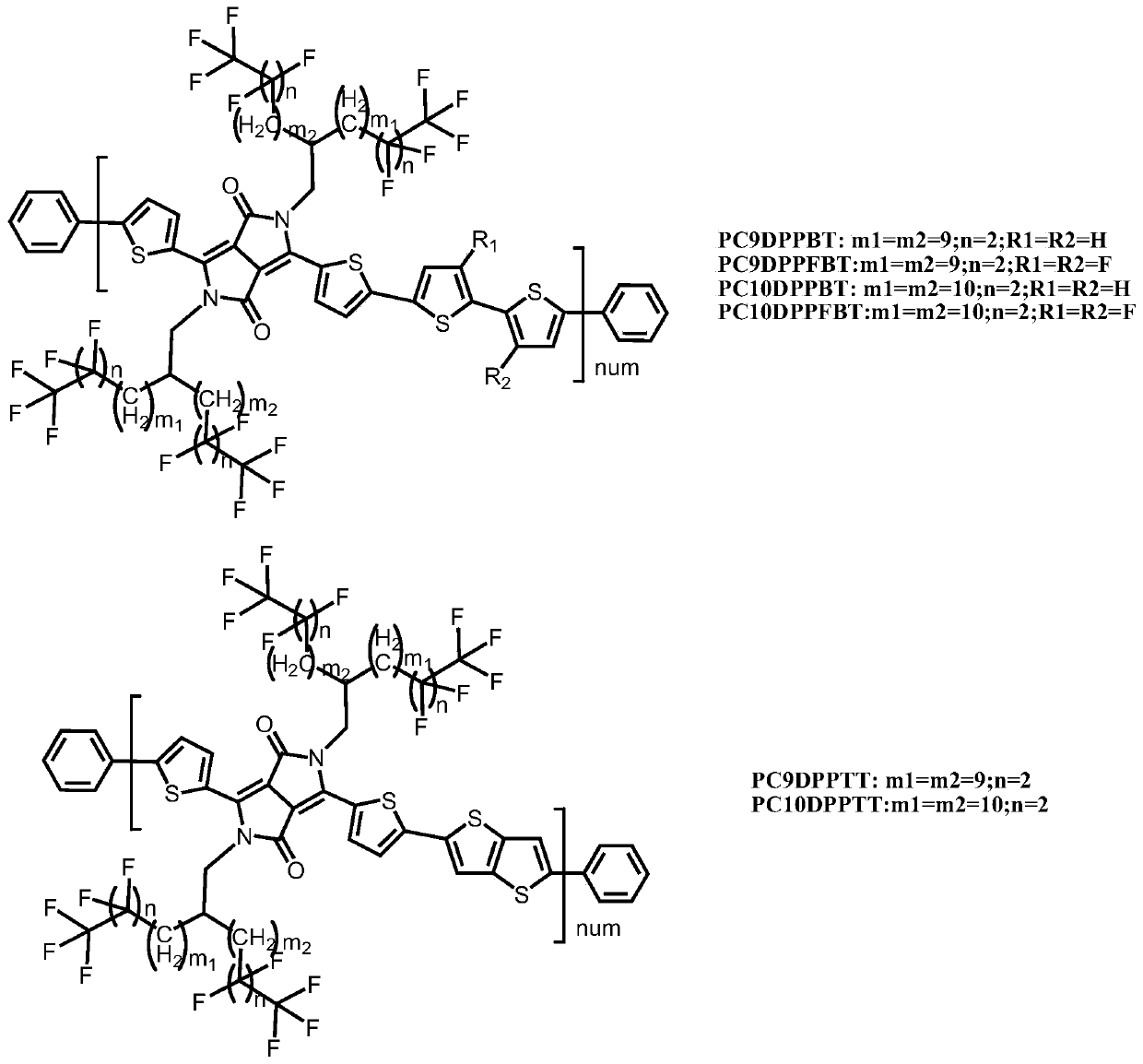
Semi-fluoroalkyl containing side chain substituted pyrrolo pyrroledione polymer as well as preparation method and application of semi-fluoroalkyl containing side chain substituted pyrrolo pyrroledione polymer
A technology of diketopyrrolopyrrole and hemifluoroalkyl, which is used in semiconductor/solid-state device manufacturing, semiconductor devices, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
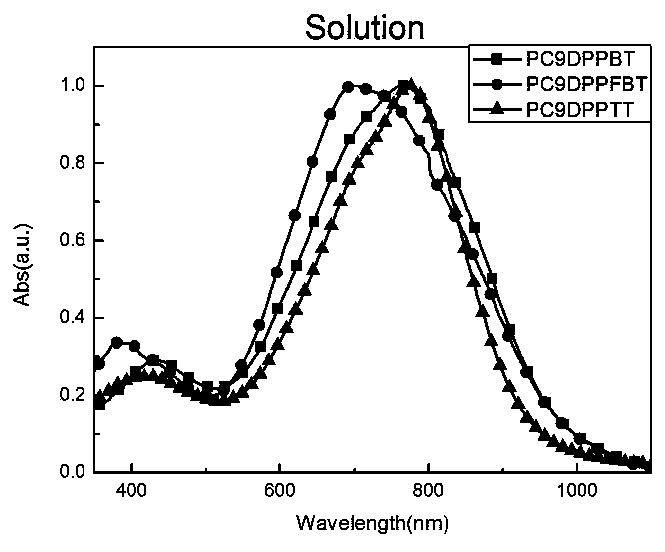
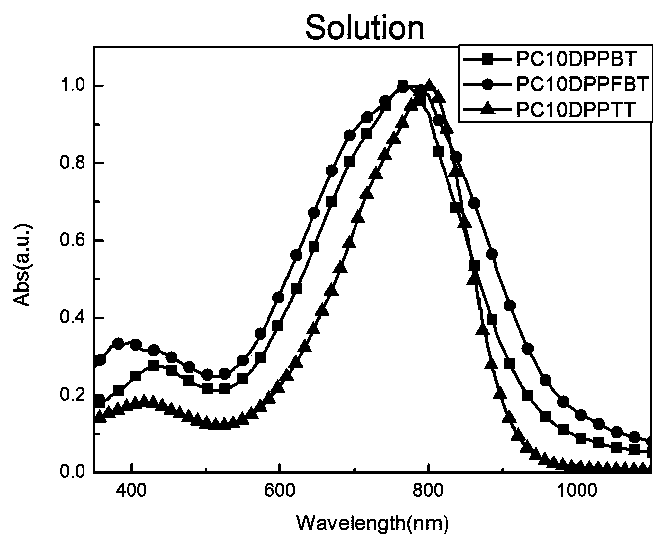
[0128] Embodiment 1, copolymer shown in preparation formula I
[0129] In the Schlenk bottle of 50mL, add the compound shown in 0.186g (0.11mmol) monomeric formula M1 (m 1 = m 2 =9; n=2), 0.049g (0.11mmol) compound 5,5-bistrimethylsilyl-2,2'-bithiophene shown in monomer formula M2 and 4mL dehydrated chlorobenzene, the reaction system adopts liquid The nitrogen cooling cycle displaces the nitrogen three times. 2.22 mg (0.0024 mmol) of tris(dibenzylideneacetone) dipalladium, 3 mg (0.01 mmol) of tri-o-tolylphosphine were added. Stir under reflux at 115°C for 48 hours. 2ml of bromobenzene was added to react overnight to complete the capping of the polymer. After cooling to room temperature, the reaction mixture was poured into 200 mL of methanol solution containing 15 mL of hydrochloric acid to settle, and then suction filtered to collect a black solid. Then use a Soxhlet extractor to separate the pure product, the washing solvent is methanol (12 hours), n-hexane (12 hours), ...
Embodiment 2
[0131] Embodiment 2, copolymer shown in preparation formula I
[0132] In the Schlenk bottle of 50mL, add the compound shown in 0.186g (0.11mmol) monomeric formula M1 (m 1 = m 2 =9; n=2), 0.058g (0.11mmol) compound (3,3'-difluoro-[2,2'-dithiophene]-5,5'-diyl)bis represented by monomer formula M2 trimethyltin and 5mL of anhydrous chlorobenzene, and the reaction system was replaced with nitrogen three times by liquid nitrogen cooling cycle. 2.22 mg (0.0024 mmol) of tris(dibenzylideneacetone) dipalladium, 3 mg (0.01 mmol) of tri-o-tolylphosphine were added. Stir under reflux at 115°C for 48 hours. 2ml of bromobenzene was added to react overnight to complete the capping of the polymer. After cooling to room temperature, the reaction mixture was poured into 200 mL of methanol solution containing 15 mL of hydrochloric acid for settling, and the black solid was collected by suction filtration. Then use a Soxhlet extractor to separate the pure product, the washing solvent is meth...
Embodiment 3
[0135] Embodiment 3, copolymer shown in preparation formula I
[0136] In the Schlenk bottle of 50mL, add the compound shown in 0.186g (0.11mmol) monomeric formula M1 (m 1 = m 2 =9; n=2), 0.052g (0.11mmol) compound 2,5-bis(trimethyltin)-thienothiophene shown in monomer formula M2 and 5mL dehydrated chlorobenzene, the reaction system was cooled by liquid nitrogen circulation Nitrogen was replaced three times. 2.22 mg (0.0024 mmol) of tris(dibenzylideneacetone) dipalladium, 3 mg (0.01 mmol) of tri-o-tolylphosphine were added. Stir under reflux at 115°C for 48 hours. 2ml of bromobenzene was added to react overnight to complete the capping of the polymer. After cooling to room temperature, the reaction mixture was poured into 200 mL of methanol solution containing 15 mL of hydrochloric acid to settle, and then suction filtered to collect a black solid. Then use a Soxhlet extractor to separate the pure product, the washing solvent is methanol (12 hours), n-hexane (12 hours), c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com