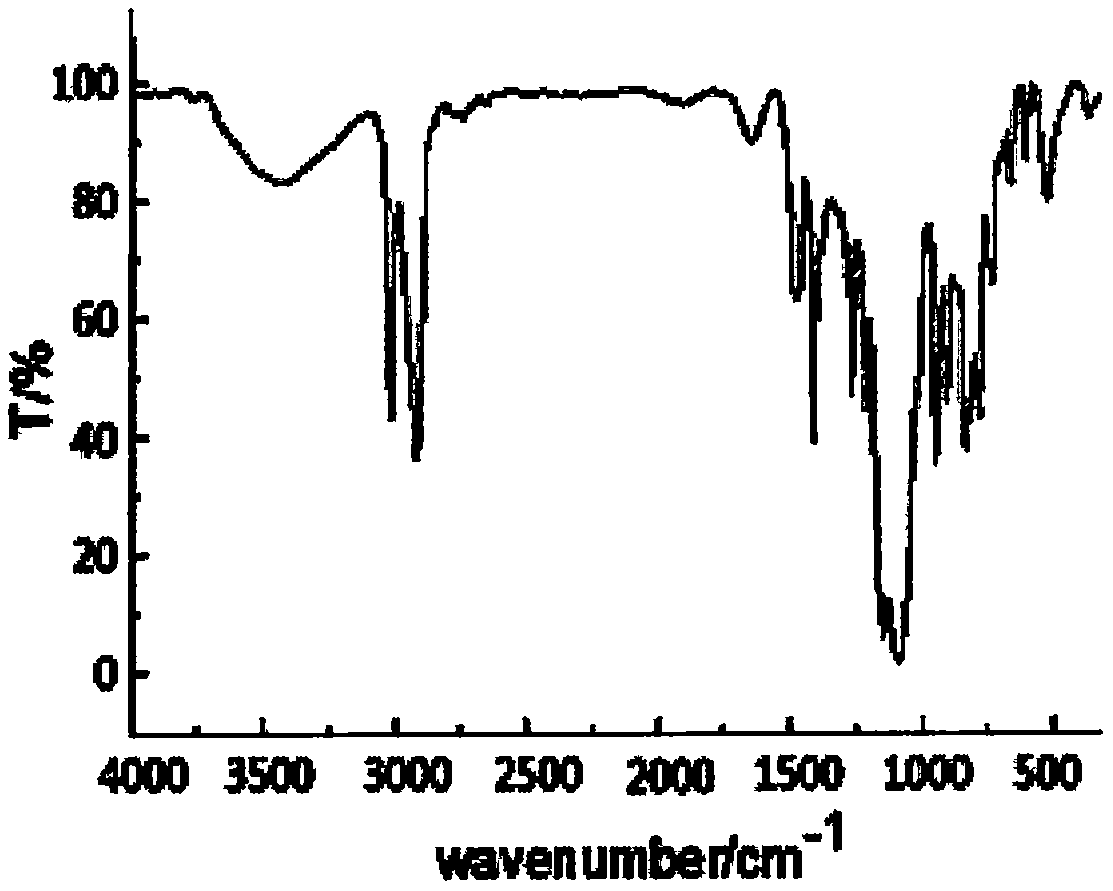
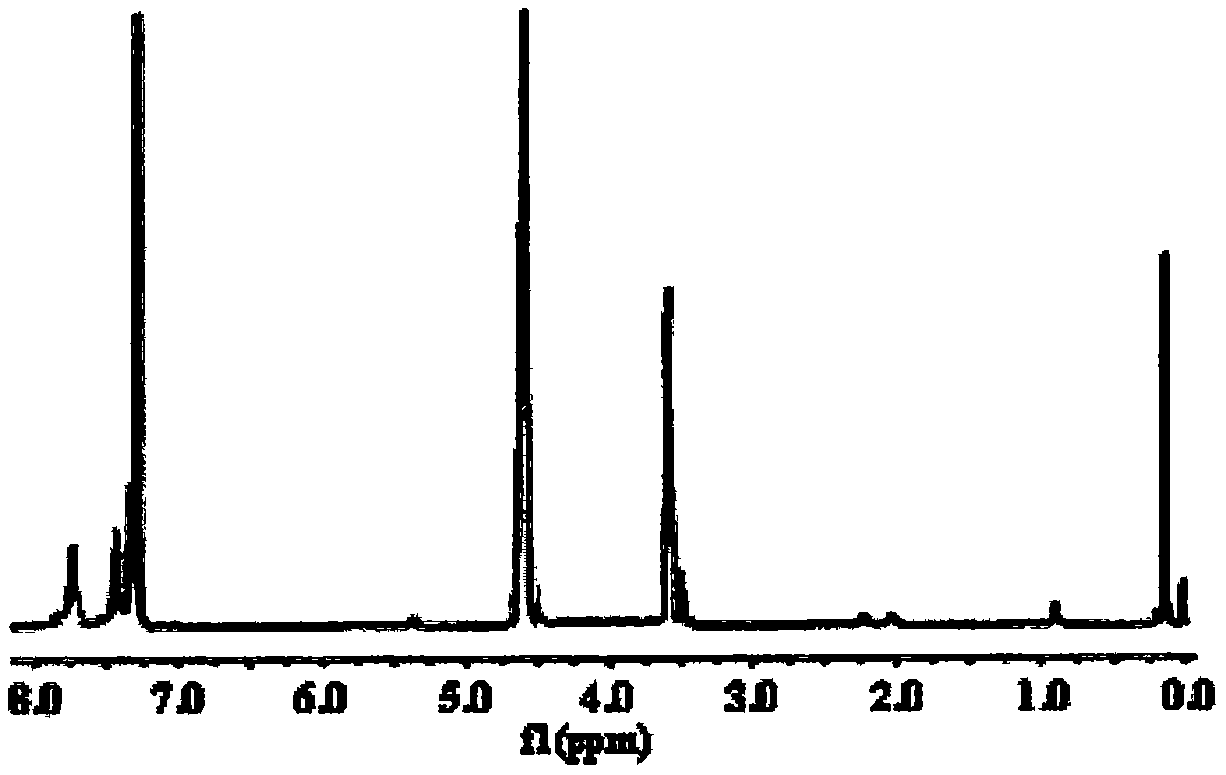
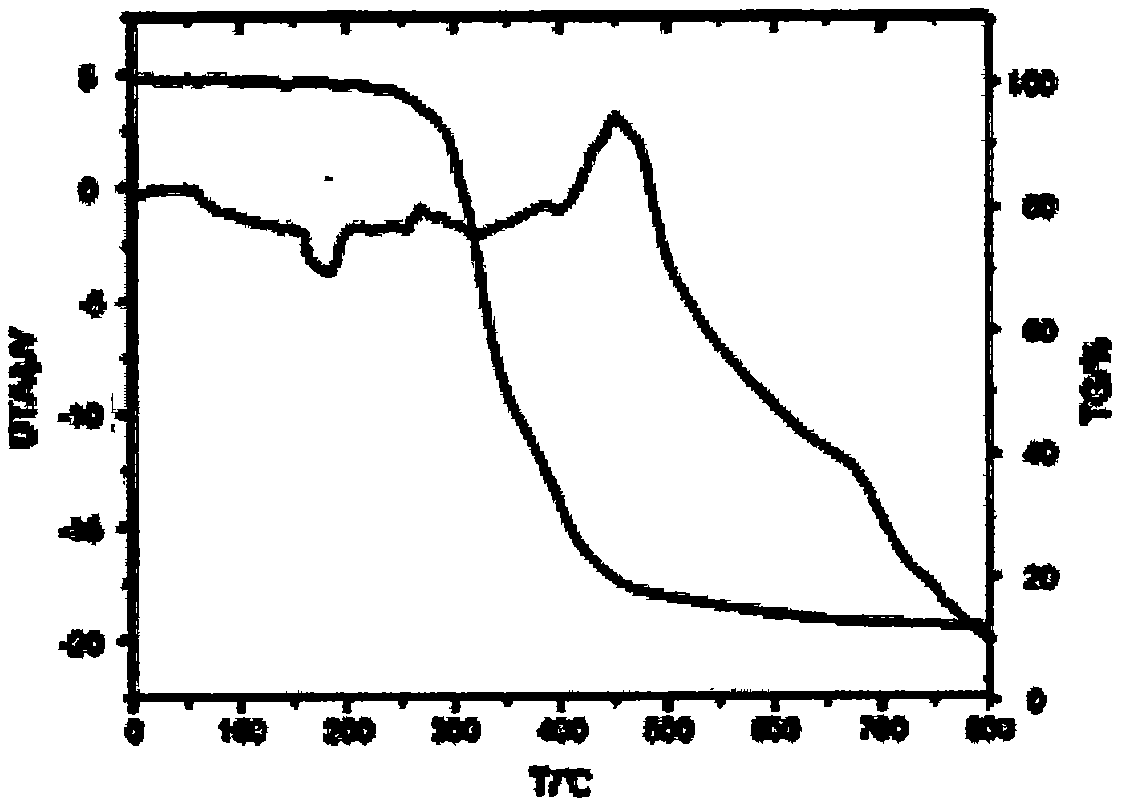
Fire retardant silicon phosphine heterocyclic methylene diphenylthioc phosphinate compound and preparation method thereof
A technology of diphenylthiophosphinic acid and heterocyclic methylene ester, which is applied in the field of flame retardant diphenylthiophosphinic acid silicon phosphine heterocyclic methylene ester compound and its preparation field, and can solve the problem of fire Threat and other issues, to achieve the effect of less investment in equipment, stable structure, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 In a 250ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, the air in the bottle was driven out with nitrogen, and 100ml of diethylene glycol dimethyl was added. Ether, 11.76g (0.06mol) trimethylolphosphine oxide cyclic ester of dimethyl silicate, after the trimethylol phosphine oxide cyclic ester of dimethyl silicate is completely dissolved, 12.65g ( 0.05mol) of diphenylthiophosphinyl chloride, control the temperature during the dropping process to not exceed 60°C, raise the temperature to 135°C after the dropwise addition, and keep the temperature for 11 hours to keep the hydrogen chloride released, adjust the pH of the reaction system to 6 with a pyridine acid-binding agent -7, Diethylene glycol dimethyl ether (recycled) was removed by distillation under reduced pressure, 90ml of ethanol was added to the crude product for heatin...
Embodiment 2
[0027] Example 2 In a 250ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, the air in the bottle was driven out with nitrogen, and 90ml of diethylene glycol dimethyl was added. Ether, 10.78g (0.055mol) trimethylolphosphine oxide cyclic ester of dimethyl silicate, after the trimethylol phosphine oxide cyclic ester of dimethyl silicate is completely dissolved, add 12.65g dropwise with stirring at 30°C (0.05mol) diphenylphosphinyl thiochloride, control the temperature during the dropwise addition to not exceed 60°C, raise the temperature to 140°C after the dropwise addition, keep the temperature for 11 hours, and adjust the reaction system with triethylamine acid-binding agent after the hydrogen chloride is released pH = 6-7, diethylene glycol dimethyl ether (recycled) was removed by distillation under reduced pressure, 90ml of ethanol was added to t...
Embodiment 3
[0028] Example 3 In a 250ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, the air in the bottle was driven out with nitrogen, and 110ml of diethylene glycol dimethyl was added. Ether, 9.80g (0.05mol) trihydroxymethylphosphine oxide cyclic ester of dimethyl silicate, after the trimethylol phosphine oxide cyclic ester of dimethyl silicate is completely dissolved, add 12.65g dropwise with stirring at 30°C (0.05mol) diphenylphosphinyl thiochloride, the dropwise addition process controls the temperature not to exceed 60°C, after the dropwise addition, the temperature is raised to 130°C, and the temperature is kept for 10 hours. After the hydrogen chloride is released, the pH of the reaction system is adjusted with a pyridine acid-binding agent. 6-7, Diethylene glycol dimethyl ether (recycled) was removed by distillation under reduced pressure, 90ml of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com