Polyhydroxyalkanoate in-vitro synthesis method
A polyhydroxyalkanoate, in vitro synthesis technology, applied in the direction of fermentation, etc., to achieve the effect of controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The polyhydroxy fatty acid copolyester P(3HB-co-2HB) of the present invention is synthesized in vitro by microorganisms, and the raw materials for in vitro synthesis mainly include the synthesis of the substrate (R)-3HB and the related enzyme ACS in the synthesis pathway of (R)-2HB Enzyme (hereinafter referred to as ACS), PCT synthetase (hereinafter referred to as PCT), ReC synthetase (hereinafter referred to as ReC) and auxiliary reagents required for the reaction. The synthetic method mainly comprises the following steps:
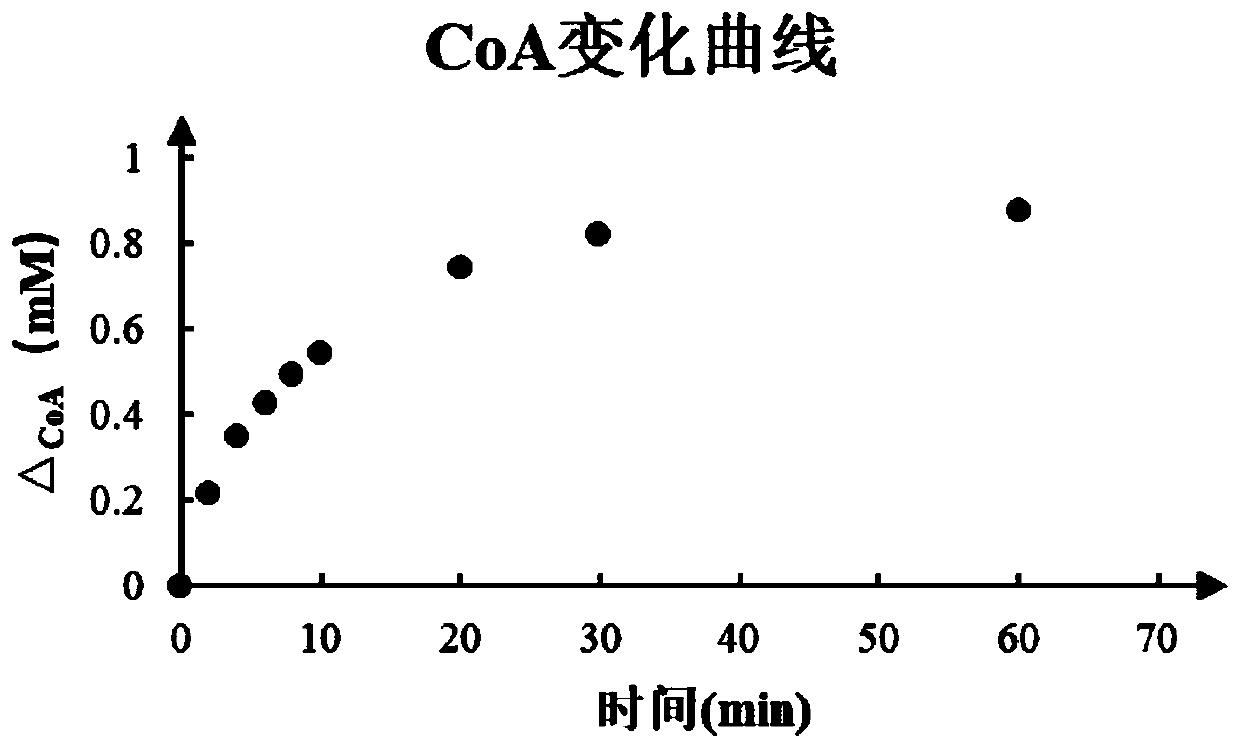
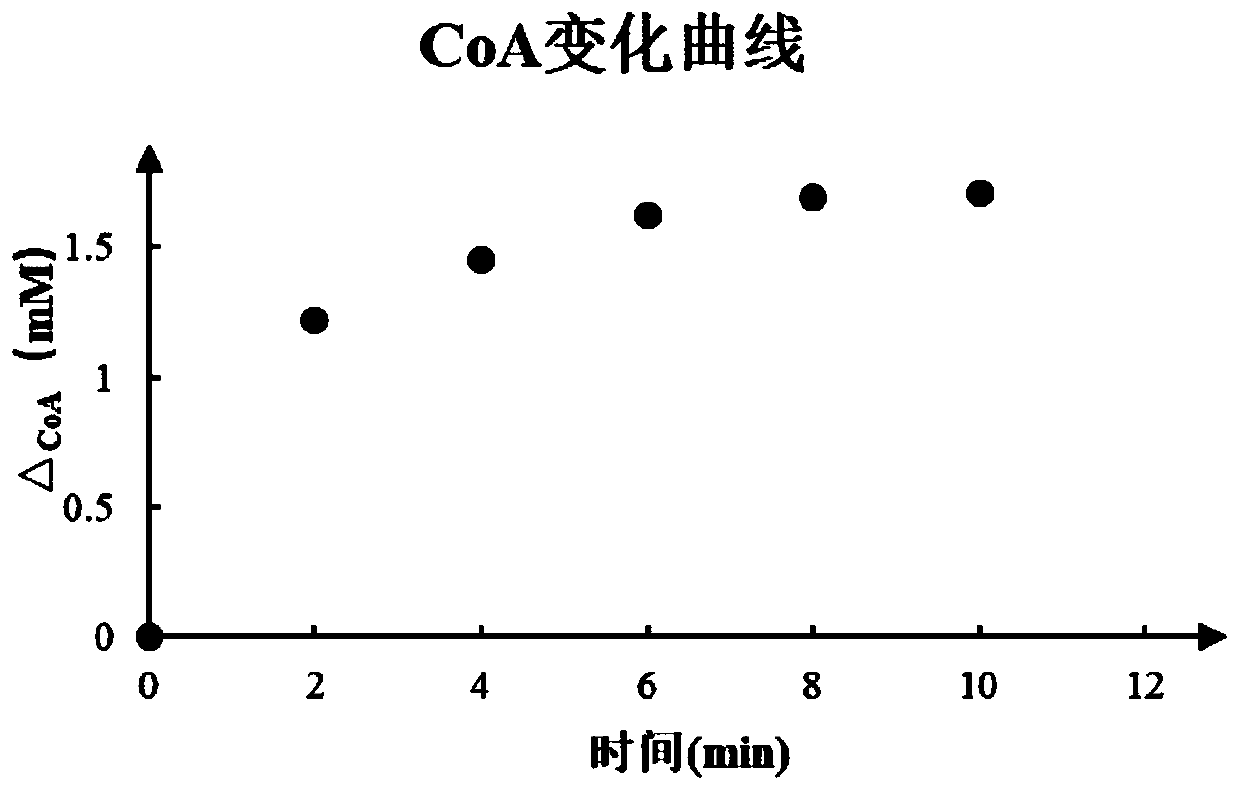
[0030] The ACS synthetase, PCT synthetase and ReC synthetase (ACS0.2mg / mL, PCT0.2mg / mL, ReC0.5mg / mL) obtained by in vitro recombinant expression. Add to the aqueous phase system containing 200mM (R)-3HB and (R)-2HB and mix (the aqueous phase system solution includes: 100mM sodium dihydrogen phosphate, 10mM magnesium chloride, 30mM ATP, 0.2mg / ml bovine serum albumin, 1mM CoA and 10mM acetic acid), at 28-31°C for 60-84h to react.
[0031] According...
specific Embodiment approach 1
[0042] The in vitro synthesis of polyhydroxy fatty acid copolyester P (3HB-co-2HB) in this embodiment comprises the following steps:
[0043]Step 1. Construction of expression strains: the pCold-acs, pQE-pct, and pCold-phaC plasmids were introduced into Escherichia coli BL21 (DE3) by thermal transformation, and the transformants were spread on the LB-Amp solid culture plate, 37 Cultivate overnight. Pick the single clone grown on the plate, inoculate it into LB-Amp liquid medium, culture it on a shaker at 37°C and 180rpm for 8 hours, extract the plasmid and perform gene sequencing verification, and the obtained sequences are all correct. The BL21 (DE3) recombinant bacteria containing pCold-acs were named BL21-ACS; the BL21 (DE3) recombinant bacteria containing pQE-pct were named BL21-PCT; the BL21 (DE3) recombinant bacteria containing pCold-phaC were named BL21-ReC.
[0044] Step 2. Induced expression of target protein: inoculate BL21-ACS, BL21-PCT, and BL21-ReC overnight act...
specific Embodiment approach 2
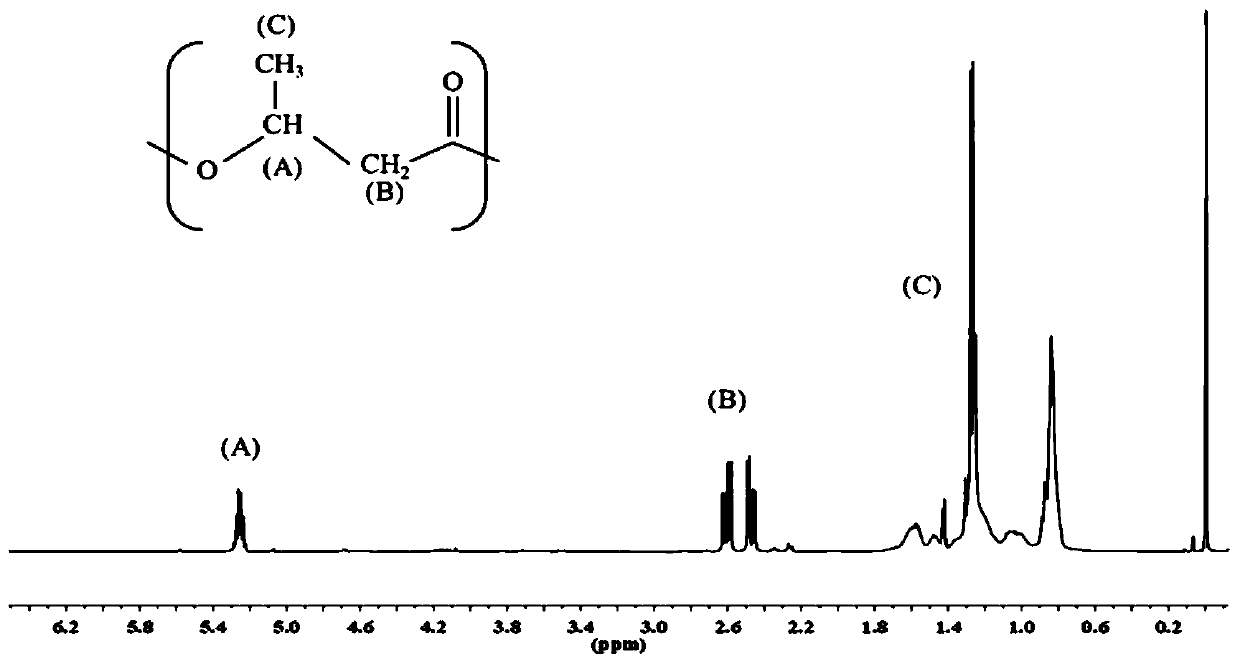
[0056] Specific embodiment 2: The difference from specific embodiment 1 is that 3HB is referred to as 2HB in the addition ratio: 3HB is added in a volume of 750 μL, 2HB is added in a volume of 250 μL, and the rest of the components are added according to Table 1. After the reaction, 4.7 mg of polymer is obtained. Hydroxy fatty acid esters. Among them, the ratio of 2HB / 3HB is 25 / 75.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com