Pyridine compound and preparation method and application thereof
A compound, pyridine technology, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve problems such as toxic side effects and affecting drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
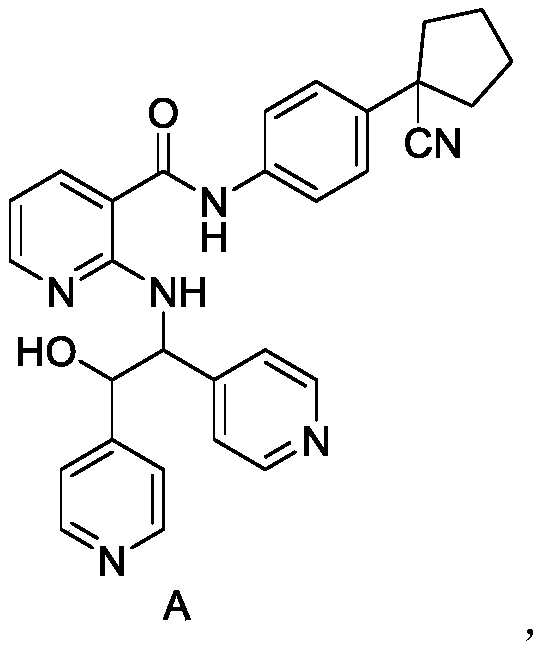
[0035] React N-[4-(1-cyanocyclopentyl)phenyl]-2-(4-pyridylmethyl)amino-3-pyridineformyl and methanesulfonic acid at 90°C, cool, filter, and the mother liquor Prepared by using a preparative liquid phase system, and isolated the compound mesylate of formula A
[0036] The preparation liquid phase condition is:
[0037] Preparative column model: 50mm inner diameter column, 10μm hydrophobic C18 filler
[0038] Detection wavelength: 220nm
[0039] Flow rate: 60ml / min
[0040] Mobile phase: A: Purified water (0.1% formic acid)
[0041] B: Acetonitrile
[0042] gradient table
[0043] time (min)
A(%)
B(%)
0
75
25
60
75
25
[0044] Mass spectrum: [M1+H] + = 505.38;
[0045] 1 H-NMR (400MHz, CD 3 OD)1.28(m,2H)1.98-2.03(m,5H),2.12-2.19(m,2H),2.43-2.48(m,2H),5.23-5.25(d,1H),5.55-5.56(d, 1H),6.63-6.66(q,1H),7.23-7.25(d,2H),7.30-7.32(d,2H), 7.50-7.52(d,2H),7.73-7.75(d,2H),8.02- 8.08(m,2H),8.31-8.32(d,2H),8.38-8.39(d,2H);
[004...
Embodiment 2
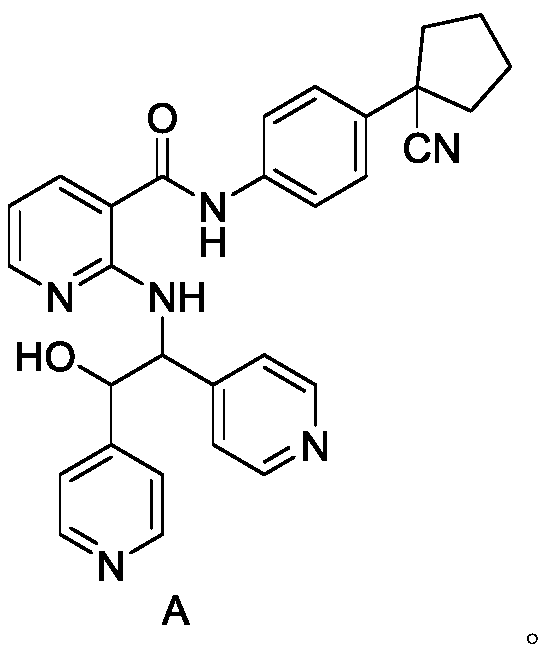
[0048] In a 250L reactor, put 9kg N-[4-(1-cyanocyclopentyl)phenyl]-2-(4-pyridylmethyl)amino-3-pyridinecarbonyl (compound C), 95% iso Add 135 L of propanol aqueous solution, then add 2.05 kg of methanesulfonic acid, heat to dissolve, press filter, cool down at 0-10°C to crystallize, filter, wash, and dry to obtain a white solid with a yield of 90%.
[0049] Related Substance Testing
[0050] Preparation of the test solution: take N-(4-(1-cyanocyclopentyl)phenyl)-2-((2-hydroxyl-1,2-bis(pyridin-4-yl)ethyl)amino ) Nicotinamide methanesulfonate 25mg, placed in a 50ml measuring bottle, dissolved in a solvent (water: acetonitrile=70:30) and diluted to the mark, shaken up;
[0051] The preparation of reference substance solution: take 25 mg of compound mesylate of formula A, place in a 50ml measuring bottle, dissolve and dilute to the scale with solvent (water: acetonitrile=70:30), get 1ml in a 100ml measuring bottle, add solvent Dilute to the mark, shake well and set aside;
[005...
Embodiment 3
[0058] The related substance detection of the tablet preparation prepared by embodiment 2 crude drug
[0059] The preparation of need testing solution: take tablet, get fine powder 45mg after grinding, add solvent (water: acetonitrile=70:30) dissolve and dilute to scale, shake up, filter for subsequent use;
[0060] The preparation of reference substance solution: take 25 mg of compound mesylate of formula A, place in a 50ml measuring bottle, dissolve and dilute to the scale with solvent (water: acetonitrile=70:30), get 1ml in a 100ml measuring bottle, add solvent Dilute to the mark, shake well and set aside;
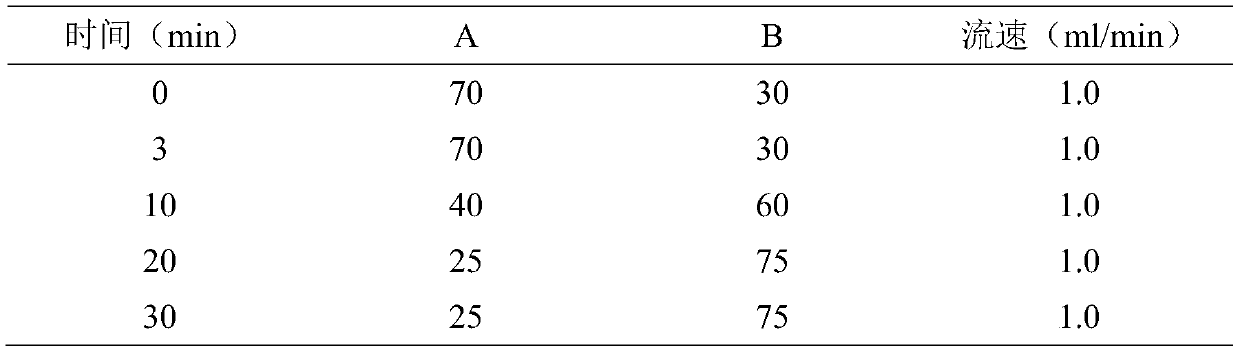
[0061] Chromatographic conditions: octadecylsilane bonded silica gel as filler, 0.01mol / L potassium dihydrogen phosphate solution as mobile phase A, acetonitrile as mobile phase B, gradient elution, detection wavelength is 260nm, gradient elution procedure is as follows :
[0062]
[0063] Measure 10 μl each of the test solution and the reference solution, inject t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com